Layer-specific integration of locomotion and sensory information in mouse barrel cortex
- PMID: 31197148
- PMCID: PMC6565743
- DOI: 10.1038/s41467-019-10564-8
Layer-specific integration of locomotion and sensory information in mouse barrel cortex
Abstract
During navigation, rodents continually sample the environment with their whiskers. How locomotion modulates neuronal activity in somatosensory cortex, and how it is integrated with whisker-touch remains unclear. Here, we compared neuronal activity in layer 2/3 (L2/3) and L5 of barrel cortex using calcium imaging in mice running in a tactile virtual reality. Both layers increase their activity during running and concomitant whisking, in the absence of touch. Fewer neurons are modulated by whisking alone. Whereas L5 neurons respond transiently to wall-touch during running, L2/3 neurons show sustained activity. Consistently, neurons encoding running-with-touch are more abundant in L2/3 and they encode the run-speed better during touch. Few neurons across layers were also sensitive to abrupt perturbations of tactile flow during running. In summary, locomotion significantly enhances barrel cortex activity across layers with L5 neurons mainly reporting changes in touch conditions and L2/3 neurons continually integrating tactile stimuli with running.
Conflict of interest statement
The authors declare no competing interests.
Figures

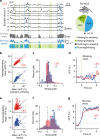
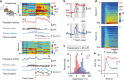
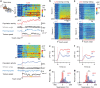


References
Publication types
MeSH terms
Grants and funding
- PMPDP3_145476/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- PZ00P3_161544/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- 31003A-149858/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- CRSII3_147660/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)/International
- 670757/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)/International
LinkOut - more resources
Full Text Sources

