Atomic-resolution imaging of surface and core melting in individual size-selected Au nanoclusters on carbon
- PMID: 31197150
- PMCID: PMC6565695
- DOI: 10.1038/s41467-019-10713-z
Atomic-resolution imaging of surface and core melting in individual size-selected Au nanoclusters on carbon
Abstract
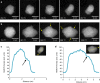
Although the changes in melting behaviour on the nanoscale have long attracted the interest of researchers, the mechanism by which nanoparticles melt remains an open problem. We report the direct observation, at atomic resolution, of surface melting in individual size-selected Au clusters (2-5 nm diameter) supported on carbon films, using an in situ heating stage in the aberration corrected scanning transmission electron microscope. At elevated temperatures the Au nanoparticles are found to form a solid core-liquid shell structure. The cluster surface melting temperatures, show evidence of size-dependent melting point suppression. The cluster core melting temperatures are significantly greater than predicted by existing models of free clusters. To explore the effect of the interaction between the clusters and the carbon substrate, we employ a very large-scale ab initio simulation approach to investigate the influence of the support. Theoretical results for surface and core melting points are in good agreement with experiment.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Schmidt M, Kusche R, von Issendorff B, Haberland H. Irregular variations in the melting point of size-selected atomic clusters. Nature. 1998;393:238–240. doi: 10.1038/30415. - DOI
-
- Pawlow P. The dependency of the melting point on the surface energy of a solid body. Z. Phys. Chem. 1909;65:545–548.
-
- Takagi M. Electron-diffraction study of liquid-solid transition of thin metal films. J. Phys. Soc. Jpn. 1954;9:359–363. doi: 10.1143/JPSJ.9.359. - DOI
Publication types
Grants and funding
- 310451/EC | EC Seventh Framework Programm | FP7 Coordination of Non-Community Research Programmes (FP7-GA - Coordination of Non-Community Research Programmes)/International
- 663830-SU165/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)/International
- 686053/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)/International
- EP/K006061/2/RCUK | Engineering and Physical Sciences Research Council (EPSRC)/International
LinkOut - more resources
Full Text Sources

