Assessment of Pharmacology, Safety, and Metabolic activity of Capsaicin Feeding in Mice
- PMID: 31197191
- PMCID: PMC6565628
- DOI: 10.1038/s41598-019-45050-0
Assessment of Pharmacology, Safety, and Metabolic activity of Capsaicin Feeding in Mice
Abstract
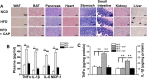
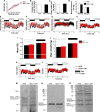
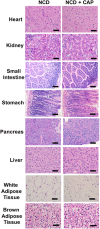
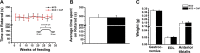
Capsaicin (CAP) activates transient receptor potential vanilloid subfamily 1 (TRPV1) to counter high-fat diet (HFD)-induced obesity. Several studies suggest that CAP induces the browning of white adipocytes in vitro or inguinal white adipose tissue (iWAT) in vivo. However, there is a lack of data on the dose-response for CAP to inhibit HFD-induced obesity. Therefore, we first performed experiments to correlate the effect of various doses of CAP to prevent HFD-induced weight gain in wild-type (WT) mice. Next, we performed a subchronic safety study in WT mice fed a normal chow diet (NCD ± CAP, 0.01% in NCD) or HFD ± CAP (0.01% in HFD) for eight months. We analyzed the expression of adipogenic and thermogenic genes and proteins in the iWAT from these mice, conducted histological studies of vital organs, measured the inflammatory cytokines in plasma and iWAT, and evaluated liver and kidney functions. The dose-response study showed that CAP, at doses above 0.001% in HFD, countered HFD-induced obesity in mice. However, no difference in the anti-obesity effect of CAP was observed at doses above 0.003% in HFD. Also, CAP, above 0.001%, enhanced the expression of sirtuin-1 and thermogenic uncoupling protein 1 (UCP-1) in the iWAT. Safety analyses suggest that CAP did not cause inflammation. However, HFD elevated plasma alanine aminotransferase and creatinine, caused iWAT hypertrophy and hepatic steatosis, and CAP reversed these. Our data suggest that CAP antagonizes HFD-induced metabolic stress and inflammation, while it does not cause any systemic toxicities and is well tolerated by mice.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

