Homeostatic pruning and activity of epidermal nerves are dysregulated in barrier-impaired skin during chronic itch development
- PMID: 31197234
- PMCID: PMC6565750
- DOI: 10.1038/s41598-019-44866-0
Homeostatic pruning and activity of epidermal nerves are dysregulated in barrier-impaired skin during chronic itch development
Abstract
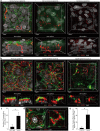
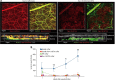
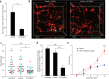
The epidermal barrier is thought to protect sensory nerves from overexposure to environmental stimuli, and barrier impairment leads to pathological conditions associated with itch, such as atopic dermatitis (AD). However, it is not known how the epidermal barrier continuously protects nerves for the sensory homeostasis during turnover of the epidermis. Here we show that epidermal nerves are contained underneath keratinocyte tight junctions (TJs) in normal human and mouse skin, but not in human AD samples or mouse models of chronic itch caused by epidermal barrier impairment. By intravital imaging of the mouse skin, we found that epidermal nerve endings were frequently extended and retracted, and occasionally underwent local pruning. Importantly, the epidermal nerve pruning took place rapidly at intersections with newly forming TJs in the normal skin, whereas this process was disturbed during chronic itch development. Furthermore, aberrant Ca2+ increases in epidermal nerves were induced in association with the disturbed pruning. Finally, TRPA1 inhibition suppressed aberrant Ca2+ increases in epidermal nerves and itch. These results suggest that epidermal nerve endings are pruned through interactions with keratinocytes to stay below the TJ barrier, and that disruption of this mechanism may lead to aberrant activation of epidermal nerves and pathological itch.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Substance P restores normal skin architecture and reduces epidermal infiltration of sensory nerve fiber in TNCB-induced atopic dermatitis-like lesions in NC/Nga mice.J Dermatol Sci. 2018 Mar;89(3):248-257. doi: 10.1016/j.jdermsci.2017.11.013. Epub 2017 Dec 1. J Dermatol Sci. 2018. PMID: 29269174
-
Deletion of mu- and kappa-opioid receptors in mice changes epidermal hypertrophy, density of peripheral nerve endings, and itch behavior.J Invest Dermatol. 2007 Jun;127(6):1479-88. doi: 10.1038/sj.jid.5700661. Epub 2006 Dec 21. J Invest Dermatol. 2007. PMID: 17185983
-
TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis.J Immunol. 2013 Dec 1;191(11):5371-82. doi: 10.4049/jimmunol.1300300. Epub 2013 Oct 18. J Immunol. 2013. PMID: 24140646 Free PMC article.
-
Peripheral itch sensitization in atopic dermatitis.Allergol Int. 2022 Jul;71(3):265-277. doi: 10.1016/j.alit.2022.04.003. Epub 2022 May 25. Allergol Int. 2022. PMID: 35624035 Review.
-
Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out?Clin Rev Allergy Immunol. 2016 Dec;51(3):263-292. doi: 10.1007/s12016-015-8488-5. Clin Rev Allergy Immunol. 2016. PMID: 25931325 Review.
Cited by
-
Skin Treatment with Detergent Induces Dermatitis with H1-Antihistamine-Refractory Itch and Upregulates IL-4 and Th17/Th22 Cytokine Gene Expression in C57BL/6 Mice.Int Arch Allergy Immunol. 2022;183(10):1040-1049. doi: 10.1159/000525656. Epub 2022 Aug 5. Int Arch Allergy Immunol. 2022. PMID: 35933977 Free PMC article.
-
Rapid structural remodeling of peripheral taste neurons is independent of taste cell turnover.PLoS Biol. 2023 Aug 31;21(8):e3002271. doi: 10.1371/journal.pbio.3002271. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37651406 Free PMC article.
-
Updated insights into the molecular pathogenesis of canine atopic dermatitis.Vet Dermatol. 2025 Aug;36(4):375-384. doi: 10.1111/vde.13300. Epub 2024 Sep 25. Vet Dermatol. 2025. PMID: 39323043 Free PMC article. Review.
-
Halting the March: Primary Prevention of Atopic Dermatitis and Food Allergies.J Allergy Clin Immunol Pract. 2020 Mar;8(3):860-875. doi: 10.1016/j.jaip.2019.12.005. J Allergy Clin Immunol Pract. 2020. PMID: 32147139 Free PMC article.
-
Skin barrier defects in atopic dermatitis: From old idea to new opportunity.Allergol Int. 2022 Jan;71(1):3-13. doi: 10.1016/j.alit.2021.11.006. Epub 2021 Dec 13. Allergol Int. 2022. PMID: 34916117 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

