Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease
- PMID: 31197263
- PMCID: PMC7136166
- DOI: 10.1038/s41581-019-0161-4
Clinical practice recommendations for growth hormone treatment in children with chronic kidney disease
Abstract
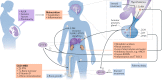
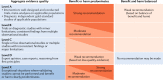
Achieving normal growth is one of the most challenging problems in the management of children with chronic kidney disease (CKD). Treatment with recombinant human growth hormone (GH) promotes longitudinal growth and likely enables children with CKD and short stature to reach normal adult height. Here, members of the European Society for Paediatric Nephrology (ESPN) CKD-Mineral and Bone Disorder (MBD), Dialysis and Transplantation working groups present clinical practice recommendations for the use of GH in children with CKD on dialysis and after renal transplantation. These recommendations have been developed with input from an external advisory group of paediatric endocrinologists, paediatric nephrologists and patient representatives. We recommend that children with stage 3-5 CKD or on dialysis should be candidates for GH therapy if they have persistent growth failure, defined as a height below the third percentile for age and sex and a height velocity below the twenty-fifth percentile, once other potentially treatable risk factors for growth failure have been adequately addressed and provided the child has growth potential. In children who have received a kidney transplant and fulfil the above growth criteria, we recommend initiation of GH therapy 1 year after transplantation if spontaneous catch-up growth does not occur and steroid-free immunosuppression is not a feasible option. GH should be given at dosages of 0.045-0.05 mg/kg per day by daily subcutaneous injections until the patient has reached their final height or until renal transplantation. In addition to providing treatment recommendations, a cost-effectiveness analysis is provided that might help guide decision-making.
Conflict of interest statement
J.B. has received speaker fees from Pfizer. B.T. has received grant support from Astellas, Novartis, Roche and Bristol-Myers Squibb and received speaker fees from Bristol-Meyers Squibb. D.H. has received research grants from Sandoz, Kyowa Kirin, Horizon and Amgen and has received speaker and/or consultant fees from Amgen, Sandoz, Kyowa Kirn, Pfizer, Merck Serono, Horizon and Chiesi. The other authors declare no competing interests.
Figures
References
-
- Franke D, et al. Growth and maturation improvement in children on renal replacement therapy over the past 20 years. Pediatr. Nephrol. 2013;28:2043–2051. - PubMed
-
- Wong CS, et al. Anthropometric measures and risk of death in children with end-stage renal disease. Am. J. Kidney Dis. 2000;36:811–819. - PubMed
-
- Furth SL, Stablein D, Fine RN, Powe NR, Fivush BA. Adverse clinical outcomes associated with short stature at dialysis initiation: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2002;109:909–913. - PubMed
-
- Mekahli D, Ledermann S, Gullett A, Rees L. Evaluation of quality of life by young adult survivors of severe chronic kidney disease in infancy. Pediatr. Nephrol. 2014;29:1387–1393. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

