The Popeye domain containing gene family encoding a family of cAMP-effector proteins with important functions in striated muscle and beyond
- PMID: 31197601
- PMCID: PMC6726836
- DOI: 10.1007/s10974-019-09523-z
The Popeye domain containing gene family encoding a family of cAMP-effector proteins with important functions in striated muscle and beyond
Abstract
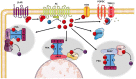
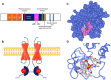
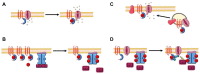
The Popeye domain containing (POPDC) gene family encodes a novel class of membrane-bound cyclic AMP effector proteins. POPDC proteins are abundantly expressed in cardiac and skeletal muscle. Consistent with its predominant expression in striated muscle, Popdc1 and Popdc2 null mutants in mouse and zebrafish develop cardiac arrhythmia and muscular dystrophy. Likewise, mutations in POPDC genes in patients have been associated with cardiac arrhythmia and muscular dystrophy phenotypes. A membrane trafficking function has been identified in this context. POPDC proteins have also been linked to tumour formation. Here, POPDC1 plays a role as a tumour suppressor by limiting c-Myc and WNT signalling. Currently, a common functional link between POPDC's role in striated muscle and as a tumour suppressor is lacking. We also discuss several alternative working models to better understand POPDC protein function.
Keywords: Atrioventricular block; Limb-girdle muscular dystrophy; Membrane trafficking; Popeye domain containing genes; Sinus node disease; Tumour suppressor; cAMP.
Figures
References
-
- Alvarez E, et al. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991;266:15277–15285. - PubMed
-
- Amunjela JN, Tucker SJ. POPDC proteins as potential novel therapeutic targets in cancer. Drug Discov Today. 2016;21:1920–1927. - PubMed
-
- Amunjela JN, Tucker SJ. POPDC1 is suppressed in human breast cancer tissues and is negatively regulated by EGFR in breast cancer cell lines. Cancer Lett. 2017;406:81–92. - PubMed

