Regulation of the Serotonergic System by Kainate in the Avian Retina
- PMID: 31197744
- PMCID: PMC11457822
- DOI: 10.1007/s10571-019-00701-8
Regulation of the Serotonergic System by Kainate in the Avian Retina
Abstract
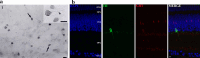
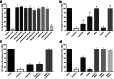
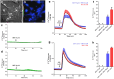
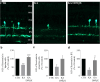
Serotonin (5-HT) has been recognized as a neurotransmitter in the vertebrate retina, restricted mainly to amacrine and bipolar cells. It is involved with synaptic processing and possibly as a mitogenic factor. We confirm that chick retina amacrine and bipolar cells are, respectively, heavily and faintly immunolabeled for 5-HT. Amacrine serotonergic cells also co-express tyrosine hydroxylase (TH), a marker of dopaminergic cells in the retina. Previous reports demonstrated that serotonin transport can be modulated by neurotransmitter receptor activation. As 5-HT is diffusely released as a neuromodulator and co-localized with other transmitters, we evaluated if 5-HT uptake or release is modulated by several mediators in the avian retina. The role of different glutamate receptors on serotonin transport and release in vitro and in vivo was also studied. We show that L-glutamate induces an inhibitory effect on [3H]5-HT uptake and this effect was specific to kainate receptor activation. Kainate-induced decrease in [3H]5-HT uptake was blocked by CNQX, an AMPA/kainate receptor antagonist, but not by MK-801, a NMDA receptor antagonist. [3H]5-HT uptake was not observed in the presence of AMPA, thus suggesting that the decrease in serotonin uptake is mediated by kainate. 5-HT (10-50 μM) had no intrinsic activity in raising intracellular Ca2+, but addition of 10 μM 5-HT decreased Ca2+ shifts induced by KCl in retinal neurons. Moreover, kainate decreased the number of bipolar and amacrine cells labeled to serotonin in chick retina. In conclusion, our data suggest a highly selective effect of kainate receptors in the regulation of serotonin functions in the retinal cells.
Keywords: Glutamate receptors; Kainate; Retina; Serotonin.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
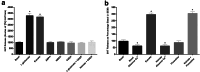
Similar articles
-
Expression of AMPA/kainate receptors during development of chick embryo retina cells: in vitro versus in vivo studies.Int J Dev Neurosci. 2002 Feb;20(1):1-9. doi: 10.1016/s0736-5748(02)00006-0. Int J Dev Neurosci. 2002. PMID: 12008069
-
Ca2+ influx through glutamate receptor-associated channels in retina cells correlates with neuronal cell death.Eur J Pharmacol. 1996 Apr 29;302(1-3):153-62. doi: 10.1016/0014-2999(96)00044-1. Eur J Pharmacol. 1996. PMID: 8791003
-
Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina.J Physiol. 2002 Jul 1;542(Pt 1):147-65. doi: 10.1113/jphysiol.2002.020305. J Physiol. 2002. PMID: 12096058 Free PMC article.
-
Characterization of receptors for glutamate and GABA in retinal neurons.Prog Neurobiol. 2004 Jun;73(2):127-50. doi: 10.1016/j.pneurobio.2004.04.002. Prog Neurobiol. 2004. PMID: 15201037 Review.
-
Adhesion molecules in establishing retinal circuitry.Curr Opin Neurobiol. 2009 Aug;19(4):389-94. doi: 10.1016/j.conb.2009.07.013. Epub 2009 Aug 5. Curr Opin Neurobiol. 2009. PMID: 19660931 Free PMC article. Review.
Cited by
-
Cell Calcium Imaging as a Reliable Method to Study Neuron-Glial Circuits.Front Neurosci. 2020 Oct 2;14:569361. doi: 10.3389/fnins.2020.569361. eCollection 2020. Front Neurosci. 2020. PMID: 33122991 Free PMC article. Review.
-
Chemical signaling in the developing avian retina: Focus on cyclic AMP and AKT-dependent pathways.Front Cell Dev Biol. 2022 Dec 9;10:1058925. doi: 10.3389/fcell.2022.1058925. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36568967 Free PMC article. Review.
References
-
- Amara SG, Kuhar MJ (1993) Neurotransmitter transporters: recent progress. Annu Rev Neurosci 16:73–93 - PubMed
-
- Beart PM (2016) Synaptic signalling and its interface with neuropathologies: snapshots from the past, present and future. J Neurochem 139(Suppl 2):76–90 - PubMed
-
- Blakely RD, Berson HE, Fremeau RT Jr, Caron MG, Peek MM, Prince HK, Bradley CC (1991) Cloning and expression of a functional serotonin transporter from rat brain. Nature 354:66–70 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

