CYFIP1 overexpression increases fear response in mice but does not affect social or repetitive behavioral phenotypes
- PMID: 31198525
- PMCID: PMC6555997
- DOI: 10.1186/s13229-019-0278-0
CYFIP1 overexpression increases fear response in mice but does not affect social or repetitive behavioral phenotypes
Abstract
Background: CYFIP1, a protein that interacts with FMRP and regulates protein synthesis and actin dynamics, is overexpressed in Dup15q syndrome as well as autism spectrum disorder (ASD). While CYFIP1 heterozygosity has been rigorously studied due to its loss in 15q11.2 deletion, Prader-Willi and Angelman syndrome, the effects of CYFIP1 overexpression, as is observed in patients with CYFIP1 duplication, are less well understood.
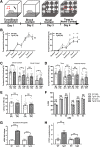
Methods: We developed and validated a mouse model of human CYFIP1 overexpression (CYFIP1 OE) using qPCR and western blot analysis. We performed a large battery of behavior testing on these mice, including ultrasonic vocalizations, three-chamber social assay, home-cage behavior, Y-maze, elevated plus maze, open field test, Morris water maze, fear conditioning, prepulse inhibition, and the hot plate assay. We also performed RNA sequencing and analysis on the basolateral amygdala.
Results: Extensive behavioral testing in CYFIP1 OE mice reveals no changes in the core behaviors related to ASD: social interactions and repetitive behaviors. However, we did observe mild learning deficits and an exaggerated fear response. Using RNA sequencing of the basolateral amygdala, a region associated with fear response, we observed changes in pathways related to cytoskeletal regulation, oligodendrocytes, and myelination. We also identified GABA-A subunit composition changes in basolateral amygdala neurons, which are essential components of the neural fear conditioning circuit.
Conclusion: Overall, this research identifies the behavioral and molecular consequences of CYFIP1 overexpression and how they contribute to the variable phenotype seen in Dup15q syndrome and in ASD patients with excess CYFIP1.
Keywords: Autism spectrum disorder (ASD); CYFIP1; Dup15q; Fear conditioning; Mouse behavior; Neurodevelopmental disorders; RNA sequencing.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures




Similar articles
-
Behavioral training rescues motor deficits in Cyfip1 haploinsufficiency mouse model of autism spectrum disorders.Transl Psychiatry. 2019 Jan 21;9(1):29. doi: 10.1038/s41398-018-0338-9. Transl Psychiatry. 2019. PMID: 30664619 Free PMC article.
-
Overexpression of Homer1a in the basal and lateral amygdala impairs fear conditioning and induces an autism-like social impairment.Mol Autism. 2016 Feb 29;7:16. doi: 10.1186/s13229-016-0077-9. eCollection 2016. Mol Autism. 2016. PMID: 26929812 Free PMC article.
-
Increased CYFIP1 dosage alters cellular and dendritic morphology and dysregulates mTOR.Mol Psychiatry. 2015 Sep;20(9):1069-78. doi: 10.1038/mp.2014.124. Epub 2014 Oct 14. Mol Psychiatry. 2015. PMID: 25311365 Free PMC article.
-
The 15q11.2 BP1-BP2 Microdeletion (Burnside-Butler) Syndrome: In Silico Analyses of the Four Coding Genes Reveal Functional Associations with Neurodevelopmental Phenotypes.Int J Mol Sci. 2020 May 6;21(9):3296. doi: 10.3390/ijms21093296. Int J Mol Sci. 2020. PMID: 32384786 Free PMC article. Review.
-
Evidence for a Contribution of the Nlgn3/Cyfip1/Fmr1 Pathway in the Pathophysiology of Autism Spectrum Disorders.Neuroscience. 2020 Oct 1;445:31-41. doi: 10.1016/j.neuroscience.2019.10.011. Epub 2019 Nov 6. Neuroscience. 2020. PMID: 31705895 Review.
Cited by
-
Orchestration of synaptic functions by WAVE regulatory complex-mediated actin reorganization.Exp Mol Med. 2023 Jun;55(6):1065-1075. doi: 10.1038/s12276-023-01004-1. Epub 2023 Jun 1. Exp Mol Med. 2023. PMID: 37258575 Free PMC article. Review.
-
Cistanches alleviates sevoflurane-induced cognitive dysfunction by regulating PPAR-γ-dependent antioxidant and anti-inflammatory in rats.J Cell Mol Med. 2020 Jan;24(2):1345-1359. doi: 10.1111/jcmm.14807. Epub 2019 Dec 4. J Cell Mol Med. 2020. PMID: 31802591 Free PMC article.
-
The effect of the demyelinating agent cuprizone on binge-like eating of sweetened palatable food in female and male C57BL/6 substrains.Appetite. 2020 Jul 1;150:104678. doi: 10.1016/j.appet.2020.104678. Epub 2020 Mar 21. Appetite. 2020. PMID: 32209386 Free PMC article.
-
Identification of disulfidptosis-related genes and subgroups in spinal cord injury.Spinal Cord. 2025 Jun;63(6):306-318. doi: 10.1038/s41393-025-01081-1. Epub 2025 May 4. Spinal Cord. 2025. PMID: 40319145
-
Rho GTPase Regulators and Effectors in Autism Spectrum Disorders: Animal Models and Insights for Therapeutics.Cells. 2020 Mar 31;9(4):835. doi: 10.3390/cells9040835. Cells. 2020. PMID: 32244264 Free PMC article. Review.
References
-
- Vorstman JA, et al. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry. 2006;11(1):1, 18–1, 28. - PubMed
-
- Finucane BM, et al. In: 15q duplication syndrome and related disorders. Adam MP, et al., editors. Seattle: GeneReviews®; 2016.

