Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation and Study of Diabetic Nephropathy with Atrasentan: what was learned about the treatment of diabetic kidney disease with canagliflozin and atrasentan?
- PMID: 31198532
- PMCID: PMC6543971
- DOI: 10.1093/ckj/sfz070
Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation and Study of Diabetic Nephropathy with Atrasentan: what was learned about the treatment of diabetic kidney disease with canagliflozin and atrasentan?
Abstract
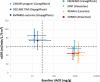
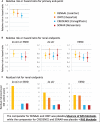
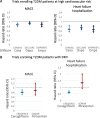
In April 2019, two major Phase 3 randomized clinical trials were published that assessed primary renal outcomes in diabetic kidney disease (DKD) in type 2 diabetes mellitus (T2DM). The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) tested an already available antidiabetic drug, canagliflozin, and the Study of Diabetic Nephropathy with Atrasentan (SONAR) tested a novel molecule, the endothelin-1 receptor blocker atrasentan, both on top of renin-angiotensin system blockade. Both trials demonstrated significant nephroprotection in patients with overt DKD (albuminuria >300 mg/g urinary creatinine) for combined primary endpoints of end-stage kidney disease (ESKD), doubling of serum creatinine or death from renal or cardiovascular causes in CREDENCE {hazard ratio [HR] 0.70 [95% confidence interval (CI) 0.59-0.82]} and ESKD and doubling of serum creatinine in SONAR [HR 0.65 (95% CI 0.49-0.88)]. Canagliflozin also decreased the secondary renal endpoint ESKD, doubling of serum creatinine or renal death [HR 0.66 (95% CI 0.53-0.81)], which was similar in nature and impact to the primary endpoint in SONAR. In addition, canagliflozin decreased a secondary endpoint of cardiovascular death or hospitalization for heart failure [HR 0.69 (95% CI 0.57-0.83)], whereas atrasentan had no significant impact on a secondary cardiovascular composite endpoint or on hospital admissions for heart failure and, despite restrictive exclusion criteria, there was a non-significant trend towards more frequent episodes of heart failure. Based on these results, canagliflozin will likely be approved for the indication of treating DKD in T2DM and the estimated glomerular filtration rate threshold for prescribing it will be lifted, whereas the future and place of atrasentan in the treatment of DKD remain unclear.
Keywords: albuminuria; atrasentan; canagliflozin; chronic kidney disease; diabetic kidney disease; endothelin; sodium-glucose cotransporter-2 (SGLT2) inhibitor.
Figures
References
-
- Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019. doi: 10.1056/NEJMoa1811744 - PubMed
-
- Heerspink HJL, Parving H-H, Andress DL. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393: 1937–1947 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw D. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WR. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

