Single-dose sodium polystyrene sulfonate for hyperkalemia in chronic kidney disease or end-stage renal disease
- PMID: 31198541
- PMCID: PMC6543963
- DOI: 10.1093/ckj/sfy063
Single-dose sodium polystyrene sulfonate for hyperkalemia in chronic kidney disease or end-stage renal disease
Abstract
Background: The use of sodium polystyrene sulfonate (SPS) for the treatment of hyperkalemia lacks sufficient efficacy data in patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD); however, use remains widespread. Recent evidence suggests that this population may be at risk for serious gastrointestinal adverse effects with SPS. Methods. We conducted a single-center retrospective cohort study. Adult patients with CKD Stages 4, 5, or ESRD maintained on renal replacement therapy with serum potassium >5 mEq/L and receipt of SPS were screened for inclusion. Our primary outcome was decrease in potassium within 24 h post-30 g oral SPS suspended in 33% sorbitol. Secondary outcomes included decrease in potassium within 24 h from 15 or 30 g SPS doses and gastrointestinal adverse events.
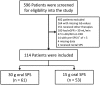
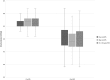
Results: Of 596 records, 114 were included for analysis. At the first serum potassium level within 24 h post-30 g oral SPS the median potassium decrease was 0.8 mEq/L [interquartile range (IQR) 0.4-1.1; P < 0.001]. At the first potassium level within 24 h post-15 or 30 g SPS, the median potassium decrease was 0.7 mEq/L (IQR 0.4-1.0; P < 0.001]. Post-SPS potassium levels occurred 14-16 h post-SPS. Gastrointestinal side effects occurred within 30 days of SPS in 5% of patients, although only two cases were classified as possibly associated.
Conclusions: The use of single-dose SPS monotherapy resulted in a significant decrease in serum potassium levels within 24 h in patients with CKD Stage 4, 5, or ESRD. However, it remains unclear if SPS is associated with an increased risk of gastrointestinal injury in this population.
Keywords: chronic kidney disease; end-stage renal disease; hyperkalemia; sodium polystyrene sulfonate.
Figures
References
-
- Centers for Disease Control and Prevention. Chronic kidney disease surveillance system – United States. https://nccd.cdc.gov/ckd/ (4 April 2018, date last accessed)
-
- KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 91–111 - PubMed
-
- KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 6
-
- Alfonzo A, Soar J, MacTier R. Clinical practice guidelines: treatment of acute hyperkalaemia in adults. UK Renal Association, 2014. https://renal.org/wp-content/uploads/2017/06/hyperkalaemia-guideline-1.pdf (10 August 2016, date last accessed)
LinkOut - more resources
Full Text Sources

