Advances of injectable hydrogel-based scaffolds for cartilage regeneration
- PMID: 31198581
- PMCID: PMC6547311
- DOI: 10.1093/rb/rbz022
Advances of injectable hydrogel-based scaffolds for cartilage regeneration
Abstract
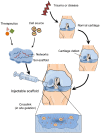
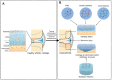
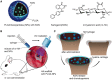
Articular cartilage is an important load-bearing tissue distributed on the surface of diarthrodial joints. Due to its avascular, aneural and non-lymphatic features, cartilage has limited self-regenerative properties. To date, the utilization of biomaterials to aid in cartilage regeneration, especially through the use of injectable scaffolds, has attracted considerable attention. Various materials, therapeutics and fabrication approaches have emerged with a focus on manipulating the cartilage microenvironment to induce the formation of cartilaginous structures that have similar properties to the native tissues. In particular, the design and fabrication of injectable hydrogel-based scaffolds have advanced in recent years with the aim of enhancing its therapeutic efficacy and improving its ease of administration. This review summarizes recent progress in these efforts, including the structural improvement of scaffolds, network cross-linking techniques and strategies for controlled release, which present new opportunities for the development of injectable scaffolds for cartilage regeneration.
Keywords: cartilage regeneration; drug delivery; injectable hydrogel; tissue engineering.
Figures

Similar articles
-
Recent advances in hydrogels for cartilage tissue engineering.Eur Cell Mater. 2017 Jan 30;33:59-75. doi: 10.22203/eCM.v033a05. Eur Cell Mater. 2017. PMID: 28138955 Free PMC article. Review.
-
Injectable hydrogels: An emerging therapeutic strategy for cartilage regeneration.Adv Colloid Interface Sci. 2023 Nov;321:103030. doi: 10.1016/j.cis.2023.103030. Epub 2023 Oct 20. Adv Colloid Interface Sci. 2023. PMID: 37907031 Review.
-
Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair.Int J Mol Sci. 2022 Aug 30;23(17):9879. doi: 10.3390/ijms23179879. Int J Mol Sci. 2022. PMID: 36077276 Free PMC article. Review.
-
Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration.Tissue Eng Part A. 2021 Sep;27(17-18):1213-1224. doi: 10.1089/ten.TEA.2020.0323. Epub 2021 Mar 1. Tissue Eng Part A. 2021. PMID: 33353462
-
Functional Biomolecule Delivery Systems and Bioengineering in Cartilage Regeneration.Curr Pharm Biotechnol. 2019;20(1):32-46. doi: 10.2174/1389201020666190206202048. Curr Pharm Biotechnol. 2019. PMID: 30727886 Review.
Cited by
-
New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers.Molecules. 2020 Mar 27;25(7):1539. doi: 10.3390/molecules25071539. Molecules. 2020. PMID: 32230990 Free PMC article. Review.
-
Porous tantalum-composited gelatin nanoparticles hydrogel integrated with mesenchymal stem cell-derived endothelial cells to construct vascularized tissue in vivo.Regen Biomater. 2021 Sep 16;8(6):rbab051. doi: 10.1093/rb/rbab051. eCollection 2021 Oct. Regen Biomater. 2021. PMID: 34603743 Free PMC article.
-
Cartilage structure-inspired nanofiber-hydrogel composite with robust proliferation and stable chondral lineage-specific differentiation function to orchestrate cartilage regeneration for artificial tracheal construction.Bioact Mater. 2025 Jan 20;47:136-151. doi: 10.1016/j.bioactmat.2025.01.007. eCollection 2025 May. Bioact Mater. 2025. PMID: 39897586 Free PMC article.
-
Recent Research Progress on Polyamidoamine-Engineered Hydrogels for Biomedical Applications.Biomolecules. 2024 May 24;14(6):620. doi: 10.3390/biom14060620. Biomolecules. 2024. PMID: 38927024 Free PMC article. Review.
-
3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration.Bioact Mater. 2021 Mar 19;6(10):3396-3410. doi: 10.1016/j.bioactmat.2021.03.013. eCollection 2021 Oct. Bioact Mater. 2021. PMID: 33842736 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

