Randomised Trial of Oral Misoprostol Versus Manual Vacuum Aspiration for the Treatment of Incomplete Abortion at a Nigerian Tertiary Hospital
- PMID: 31198594
- PMCID: PMC6544063
- DOI: 10.18295/squmj.2019.19.01.008
Randomised Trial of Oral Misoprostol Versus Manual Vacuum Aspiration for the Treatment of Incomplete Abortion at a Nigerian Tertiary Hospital
Abstract
Objectives: This study aimed to compare the efficacy of oral misoprostol with manual vacuum aspiration (MVA) in first trimester incomplete abortions.
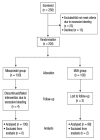
Methods: This randomised controlled trial study was conducted at the University of Ilorin Teaching Hospital, Ilorin, Nigeria between April 2014 and November 2015. Pregnant women who presented with clinical features of incomplete abortion at a gestational age of 13 weeks or less were included. Patients who had profuse vaginal bleeding, an intrauterine device in situ, signs of pelvic infections or who were younger than 18 years old and had no accompanying adults to give informed consent were excluded. A total of 200 participants were randomly and equally allocated to either the MVA or misoprostol treatment group. The treatment group were given 600 μg of misoprostol orally. The primary outcome measure was complete uterine evacuation, while secondary outcome measures included the need for additional surgical evacuation for failed treatment, adverse effects/complications, acceptability of and satisfaction with the treatment.
Results: Both misoprostol and MVA had high complete evacuation rates, yet MVA was significantly higher (99% versus 83%, relative risk [RR]: 0.84, confidence interval [CI]: 0.766-0.918; P <0.001). Significantly more women in the misoprostol group required additional MVA for failed treatment than in the MVA treatment group (17% versus 1%, RR: 16.67, CI: 2.260-12.279; P <0.001). No significant difference was found between the misoprostol and MVA treatment groups in terms of satisfaction (92.7% versus 89.8%, RR: 1.04, CI: 0.946-1.127; P = 0.473).
Conclusion: Treatments with misoprostol and MVA had high complete uterine evacuation rates, as well as high rates of acceptability and satisfaction. However, MVA had a significantly higher complete evacuation rate than misoprostol.
Keywords: Abortion Techniques; First Trimester; Incomplete Abortion; Misoprostol; Nigeria.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare no conflicts of interest.
Figures
Similar articles
-
Misoprostol versus manual vacuum aspiration for treatment of first-trimester incomplete miscarriage in a low-resource setting: A randomized controlled trial.Niger J Clin Pract. 2020 May;23(5):638-646. doi: 10.4103/njcp.njcp_379_19. Niger J Clin Pract. 2020. PMID: 32367870 Clinical Trial.
-
Is misoprostol a safe, effective and acceptable alternative to manual vacuum aspiration for postabortion care? Results from a randomised trial in Burkina Faso, West Africa.BJOG. 2007 Nov;114(11):1368-75. doi: 10.1111/j.1471-0528.2007.01468.x. Epub 2007 Sep 5. BJOG. 2007. PMID: 17803715 Clinical Trial.
-
A randomized controlled trial of 400-μg sublingual misoprostol versus manual vacuum aspiration for the treatment of incomplete abortion in two Egyptian hospitals.Int J Gynaecol Obstet. 2010 Nov;111(2):131-5. doi: 10.1016/j.ijgo.2010.06.021. Int J Gynaecol Obstet. 2010. PMID: 20801444 Clinical Trial.
-
Efficacy, Safety, and Acceptability of Misoprostol in the Treatment of Incomplete Miscarriage: A Systematic Review and Meta-analysis.Rev Bras Ginecol Obstet. 2023 Dec;45(12):e808-e817. doi: 10.1055/s-0043-1776029. Epub 2023 Dec 23. Rev Bras Ginecol Obstet. 2023. PMID: 38141602 Free PMC article.
-
Expectant, medical, or surgical management of first-trimester miscarriage: a meta-analysis.Obstet Gynecol. 2005 May;105(5 Pt 1):1104-13. doi: 10.1097/01.AOG.0000158857.44046.a4. Obstet Gynecol. 2005. PMID: 15863551 Review.
Cited by
-
"I will never wish this pain to even my worst enemy": Lived experiences of pain associated with manual vacuum aspiration during post-abortion care in Kenya.PLoS One. 2023 Aug 24;18(8):e0289689. doi: 10.1371/journal.pone.0289689. eCollection 2023. PLoS One. 2023. PMID: 37619217 Free PMC article.
-
Women's perceptions of and experiences with the use of misoprostol for treatment of incomplete abortion in central Malawi: a mixed methods study.Reprod Health. 2023 Feb 2;20(1):26. doi: 10.1186/s12978-022-01549-w. Reprod Health. 2023. PMID: 36732793 Free PMC article.
-
A comparison of misoprostol with and without methylergometrine and oxytocin in outpatient medical abortion: a phase III randomized controlled trial.BMC Res Notes. 2023 Oct 5;16(1):257. doi: 10.1186/s13104-023-06509-6. BMC Res Notes. 2023. PMID: 37798748 Free PMC article. Clinical Trial.
-
Proportion and factors associated with intra-procedural pain among women undergoing manual vacuum aspiration for incomplete abortion at Mbarara Regional Referral Hospital, Uganda.Pan Afr Med J. 2024 Nov 6;49:63. doi: 10.11604/pamj.2024.49.63.39955. eCollection 2024. Pan Afr Med J. 2024. PMID: 39958568 Free PMC article.
-
A Stalled Revolution? Misoprostol and the Pharmaceuticalization of Reproductive Health in Francophone Africa.Front Sociol. 2021 Apr 12;6:590556. doi: 10.3389/fsoc.2021.590556. eCollection 2021. Front Sociol. 2021. PMID: 33954164 Free PMC article.
References
-
- Adefuye PO, Sule-Odu AO, Olatunji AO, Lamina MA, Oladapo OT. Maternal deaths from induced abortions. Trop J Obstet Gynaecol. 2003;20:101–4. doi: 10.4314/tjog.v20i2.14410. - DOI
-
- World Health Organization. Safe abortion: Technical and policy guidance for health systems. [Accessed: Nov 2018]. From: apps.who.int/iris/bitstream/handle/10665/70914/9789241548434_eng.pdf?seq.... - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous