Protease-activated receptor 2 activates CRAC-mediated Ca2+ influx to cause prostate smooth muscle contraction
- PMID: 31198907
- PMCID: PMC6563600
- DOI: 10.1096/fba.2018-00024
Protease-activated receptor 2 activates CRAC-mediated Ca2+ influx to cause prostate smooth muscle contraction
Abstract
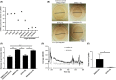
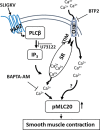
Protease activated receptor 2 (PAR2) is a G-protein coupled receptor that contributes to prostate fibrosis and lower urinary tract symptoms (LUTS). In addition to fibrosis, aberrant smooth muscle tone in the prostate has been hypothesized to play a role. We therefore examined PAR2 expression in primary human prostate smooth muscle cells (PSMC) and studied the downstream signaling effects of PAR2 activation. Signaling pathways involved in the process were assessed using the PAR2 activating peptide SLIGKV-NH2. We show that PAR2 is expressed in PSMC and that PAR2 activation mediates a biphasic elevation in intracellular Ca2+ and phosphorylation of myosin light chain 20 (MLC20), causing cellular contraction as assessed in a gel contraction assay. Intracellular Ca2+ flux was inhibited by a phosphoinositide hydrolysis inhibitor, U73122, showing a requirement for phospholipase C β (PLCβ) activation. PSMC expressed mRNA for L-type voltage dependent Ca2+ channels (VDCC) as well as Ca2+ release activated channels (CRAC), a hitherto unreported finding. Secondary intracellular Ca2+ oscillations were abrogated only by BTP2, the CRAC channel inhibitor, but not by nifedipine, an inhibitor of VDCC. These data suggest that, PAR2 activation and subsequent Ca2+ entry through CRAC channels are important mechanisms in prostate smooth muscle contraction.
Keywords: G-protein coupled receptor; PAR2; calcium channels; phospholipase; prostate; smooth muscle.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
Similar articles
-
The signaling of protease-activated receptor-2 activating peptide-induced contraction in cat esophageal smooth muscle cells.Arch Pharm Res. 2017 Dec;40(12):1443-1454. doi: 10.1007/s12272-017-0975-1. Epub 2017 Nov 2. Arch Pharm Res. 2017. PMID: 29098568
-
Store-Operated Ca2+ Release-Activated Ca2+ Channels Regulate PAR2-Activated Ca2+ Signaling and Cytokine Production in Airway Epithelial Cells.J Immunol. 2015 Sep 1;195(5):2122-33. doi: 10.4049/jimmunol.1500396. Epub 2015 Aug 3. J Immunol. 2015. PMID: 26238490 Free PMC article.
-
Expression and function of proteinase-activated receptor 2 in human bronchial smooth muscle.Am J Respir Crit Care Med. 2001 Oct 1;164(7):1276-81. doi: 10.1164/ajrccm.164.7.2101157. Am J Respir Crit Care Med. 2001. PMID: 11673222
-
Signal-transduction pathways that regulate smooth muscle function I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles.Am J Physiol Gastrointest Liver Physiol. 2005 Mar;288(3):G407-16. doi: 10.1152/ajpgi.00398.2004. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 15701619 Review.
-
Role of Ca2+ signaling in the regulation of endothelial permeability.Vascul Pharmacol. 2002 Nov;39(4-5):173-85. doi: 10.1016/s1537-1891(03)00007-7. Vascul Pharmacol. 2002. PMID: 12747958 Review.
Cited by
-
Mast cell function in prostate inflammation, fibrosis, and smooth muscle cell dysfunction.Am J Physiol Renal Physiol. 2021 Oct 1;321(4):F466-F479. doi: 10.1152/ajprenal.00116.2021. Epub 2021 Aug 23. Am J Physiol Renal Physiol. 2021. PMID: 34423679 Free PMC article.
-
Endothelial PAR2 activation evokes resistance artery relaxation.J Cell Physiol. 2023 Apr;238(4):776-789. doi: 10.1002/jcp.30973. Epub 2023 Feb 15. J Cell Physiol. 2023. PMID: 36791026 Free PMC article.
-
Effects of shRNA-mediated silencing of PDE5A3 on intracellular cGMP and free Ca2+ levels and human prostate smooth muscle cell proliferation from benign prostatic hyperplasia.Exp Ther Med. 2021 Apr;21(4):322. doi: 10.3892/etm.2021.9753. Epub 2021 Feb 5. Exp Ther Med. 2021. PMID: 33732295 Free PMC article.
References
-
- Nystedt S, Larsson A‐K, Åberg H, Sundelin J. The mouse proteinase‐activated receptor‐2 cDNA and gene molecular cloning and functional expression. J Biol Chem. 1995;270:5950‐5955. - PubMed
-
- Cottrell GS, Amadesi S, Schmidlin F, Bunnett N. Protease‐activated receptor 2: activation, signalling and function. Biochem Soc Trans. 2003;31:1191‐1197. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
