Exogenous H2S mitigates myocardial fibrosis in diabetic rats through suppression of the canonical Wnt pathway
- PMID: 31198980
- PMCID: PMC6605697
- DOI: 10.3892/ijmm.2019.4237
Exogenous H2S mitigates myocardial fibrosis in diabetic rats through suppression of the canonical Wnt pathway
Abstract
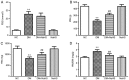
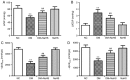
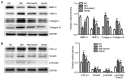
Hydrogen sulfide (H2S) has antifibrotic activity in the kidneys, heart, lungs, and other organs. The present study investigated the protective activity of exogenous H2S against myocardial fibrosis in a rat model of diabetes. Animals were assigned to normal control, diabetes mellitus (DM), DM + sodium hydrosulfide (NaHS; DM + NaHS) and NaHS groups. Fasting blood glucose (FBG), cardiac function and hydroxyproline were monitored. Heart histomorphology and ultrastructure were additionally evaluated. Wnt1‑inducible signaling pathway protein (WISP)‑1 protein expression in the myocardium was determined by immunohistochemical staining. Matrix metalloprotease (MMP)‑2, tissue inhibitor of metalloproteinase (TIMP)‑2, collagens, and canonical Wnt and transforming growth factor (TGF)‑β1/SMAD family member 3 (Smad3) pathway‑related proteins were assessed by western blotting. Cardiac function was decreased, and myocardial injury, hypertrophy and fibrosis were increased in the diabetes model rats. MMP‑2 expression was decreased, and the expressions of WISP‑1, TIMP‑2, collagens, and canonical Wnt and TGF‑β1/Smad3 pathway‑related proteins were increased in the myocardia of the diabetes model rats. The present results indicated that the canonical Wnt pathway promoted diabetic myocardial fibrosis by upregulating the TGF‑β1/Smad3 pathway. Except for FBG, exogenous H2S ameliorated the changes in diabetes‑associated indices in rats in the DM + NaHS group. The results are consistent with H2S protection of streptozotocin‑induced myocardial fibrosis in the diabetes model rats by downregulation of the canonical Wnt and TGF‑β1/Smad3 pathway and decreased myocardial collagen deposition.
Figures




Similar articles
-
Hydrogen Sulfide as a Potential Alternative for the Treatment of Myocardial Fibrosis.Oxid Med Cell Longev. 2020 Jan 23;2020:4105382. doi: 10.1155/2020/4105382. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32064023 Free PMC article. Review.
-
H2S improves renal fibrosis in STZ-induced diabetic rats by ameliorating TGF-β1 expression.Ren Fail. 2017 Nov;39(1):265-272. doi: 10.1080/0886022X.2016.1257433. Epub 2016 Nov 24. Ren Fail. 2017. PMID: 27882817 Free PMC article.
-
Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway.Int J Mol Med. 2018 Apr;41(4):1867-1876. doi: 10.3892/ijmm.2018.3419. Epub 2018 Jan 23. Int J Mol Med. 2018. PMID: 29393353 Free PMC article.
-
Effects of hydrogen sulfide on myocardial fibrosis in diabetic rats: Changes in matrix metalloproteinases parameters.Biomed Mater Eng. 2015;26 Suppl 1:S2033-9. doi: 10.3233/BME-151508. Biomed Mater Eng. 2015. PMID: 26405980
-
Gasotransmitters: Potential Therapeutic Molecules of Fibrotic Diseases.Oxid Med Cell Longev. 2021 Sep 20;2021:3206982. doi: 10.1155/2021/3206982. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34594474 Free PMC article. Review.
Cited by
-
Hydrogen Sulfide Attenuates High Glucose-induced Myocardial Injury in Rat Cardiomyocytes by Suppressing Wnt/beta-catenin Pathway.Curr Med Sci. 2019 Dec;39(6):938-946. doi: 10.1007/s11596-019-2120-5. Epub 2019 Dec 16. Curr Med Sci. 2019. PMID: 31845225
-
Protective effect of exogenous hydrogen sulfide on diaphragm muscle fibrosis in streptozotocin-induced diabetic rats.Exp Biol Med (Maywood). 2020 Aug;245(14):1280-1289. doi: 10.1177/1535370220931038. Epub 2020 Jun 3. Exp Biol Med (Maywood). 2020. PMID: 32493122 Free PMC article.
-
Hydrogen Sulfide as a Potential Alternative for the Treatment of Myocardial Fibrosis.Oxid Med Cell Longev. 2020 Jan 23;2020:4105382. doi: 10.1155/2020/4105382. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32064023 Free PMC article. Review.
-
A nutraceutical strategy for downregulating TGFβ signalling: prospects for prevention of fibrotic disorders, including post-COVID-19 pulmonary fibrosis.Open Heart. 2021 Apr;8(1):e001663. doi: 10.1136/openhrt-2021-001663. Open Heart. 2021. PMID: 33879509 Free PMC article. No abstract available.
-
Epigallocatechin-3-gallate attenuates myocardial fibrosis in diabetic rats by activating autophagy.Exp Biol Med (Maywood). 2022 Sep;247(17):1591-1600. doi: 10.1177/15353702221110646. Epub 2022 Jul 14. Exp Biol Med (Maywood). 2022. PMID: 35833541 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

