RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review)
- PMID: 31198981
- PMCID: PMC6658002
- DOI: 10.3892/ijmm.2019.4244
RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review)
Abstract
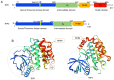
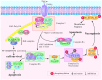
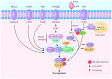
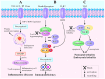
Necroptosis is a type of programmed cell death with necrotic morphology, occurring in a variety of biological processes, including inflammation, immune response, embryonic development and metabolic abnormalities. The current nomenclature defines necroptosis as cell death mediated by signal transduction from receptor‑interacting serine/threonine kinase (RIP) 1 to RIP3 (hereafter called RIP1/RIP3). However, RIP3‑dependent cell death would be a more precise definition of necroptosis. RIP3 is indispensable for necroptosis, while RIP1 is not consistently involved in the signal transduction. Notably, deletion of RIP1 even promotes RIP3‑mediated necroptosis under certain conditions. Necroptosis was previously thought as an alternate process of cell death in case of apoptosis inhibition. Currently, necroptosis is recognized to serve a pivotal role in regulating various physiological processes. Of note, it mediates a variety of human diseases, such as ischemic brain injury, immune system disorders and cancer. Targeting and inhibiting necroptosis, therefore, has the potential to be used for therapeutic purposes. To date, research has elucidated the suppression of RIP1/RIP3 via effective inhibitors and highlighted their potential application in disease therapy. The present review focused on the molecular mechanisms of RIP1/RIP3‑mediated necroptosis, explored the functions of RIP1/RIP3 in necroptosis, discussed their potential as a novel therapeutic target for disease therapy, and provided valuable suggestions for further study in this field.
Figures
Similar articles
-
Cytosolic calcium mediates RIP1/RIP3 complex-dependent necroptosis through JNK activation and mitochondrial ROS production in human colon cancer cells.Free Radic Biol Med. 2017 Jul;108:433-444. doi: 10.1016/j.freeradbiomed.2017.04.010. Epub 2017 Apr 14. Free Radic Biol Med. 2017. PMID: 28414098
-
RIP1, RIP3, and MLKL Contribute to Cell Death Caused by Clostridium perfringens Enterotoxin.mBio. 2019 Dec 17;10(6):e02985-19. doi: 10.1128/mBio.02985-19. mBio. 2019. PMID: 31848291 Free PMC article.
-
Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway.Invest New Drugs. 2020 Feb;38(1):50-59. doi: 10.1007/s10637-019-00764-w. Epub 2019 Mar 28. Invest New Drugs. 2020. PMID: 30924024 Free PMC article.
-
Connections between various trigger factors and the RIP1/ RIP3 signaling pathway involved in necroptosis.Asian Pac J Cancer Prev. 2013;14(12):7069-74. doi: 10.7314/apjcp.2013.14.12.7069. Asian Pac J Cancer Prev. 2013. PMID: 24460252 Review.
-
Necroptosis: an emerging form of programmed cell death.Crit Rev Oncol Hematol. 2012 Jun;82(3):249-58. doi: 10.1016/j.critrevonc.2011.08.004. Epub 2011 Oct 1. Crit Rev Oncol Hematol. 2012. PMID: 21962882 Review.
Cited by
-
Loss of Ripk3 attenuated neutrophil accumulation in a lipopolysaccharide-induced zebrafish inflammatory model.Cell Death Discov. 2022 Feb 26;8(1):88. doi: 10.1038/s41420-022-00891-z. Cell Death Discov. 2022. PMID: 35220408 Free PMC article.
-
Application of Regulatory Cell Death in Cancer: Based on Targeted Therapy and Immunotherapy.Front Immunol. 2022 Mar 10;13:837293. doi: 10.3389/fimmu.2022.837293. eCollection 2022. Front Immunol. 2022. PMID: 35359956 Free PMC article. Review.
-
Nanoencapsulation of Docetaxel Induces Concurrent Apoptosis and Necroptosis in Human Oral Cancer Cells (SCC-9) via TNF-α/RIP1/RIP3 Pathway.Indian J Clin Biochem. 2023 Jul;38(3):351-360. doi: 10.1007/s12291-022-01055-7. Epub 2022 Aug 9. Indian J Clin Biochem. 2023. PMID: 37234186 Free PMC article.
-
Expression of HIF1α in intestinal epithelium restricts arthritis inflammation by inhibiting RIPK3-induced cell death machinery.Ann Rheum Dis. 2024 Jul 15;83(8):984-997. doi: 10.1136/ard-2023-224491. Ann Rheum Dis. 2024. PMID: 38503474 Free PMC article.
-
Differential Co-Expression Network Analysis Reveals Key Hub-High Traffic Genes as Potential Therapeutic Targets for COVID-19 Pandemic.Front Immunol. 2021 Dec 15;12:789317. doi: 10.3389/fimmu.2021.789317. eCollection 2021. Front Immunol. 2021. PMID: 34975885 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

