Novel strategy of ovarian cancer implantation: Pre-invasive growth of fibrin-anchored cells with neovascularization
- PMID: 31199029
- PMCID: PMC6676109
- DOI: 10.1111/cas.14098
Novel strategy of ovarian cancer implantation: Pre-invasive growth of fibrin-anchored cells with neovascularization
Abstract
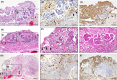
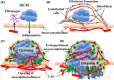
Although direct adhesion of cancer cells to the mesothelial cell layer is considered to be a key step for peritoneal invasion of ovarian cancer cell masses (OCM), we recently identified a different strategy for the peritoneal invasion of OCM. In 6 out of 20 cases of ovarian carcinoma, extraperitoneal growth of the OCM was observed along with the neovascularization of feeding vessels, which connect the intraperitoneal host stroma and extraperitoneal lesions through the intact mesothelial cell layer. As an early step, the OCMs anchor in the extraperitoneal fibrin networks and then induce the migration of CD34-positive and vascular endothelial growth factor A (VEGF-A)-positive endothelial cells, constructing extraperitoneal vascular networks around the OCM. During the extraperitoneal growth of OCM, podoplanin-positive and α smooth muscle actin (αSMA)-positive cancer-associated fibroblasts (CAF) appears. In more advanced lesions, the boundary line of mesothelial cells disappears around the insertion areas of feeding vessels and then extraperitoneal and intraperitoneal stroma are integrated, enabling the OCM to invade the host stroma, being associated with CAF. In addition, tissue factors (TF) are strongly detected around these peritoneal implantation sites and their levels in ascites were higher than that in blood. These findings demonstrate the presence of neovascularization around fibrin net-anchored OCMs on the outer side of the intact peritoneal surface, suggesting a novel strategy for peritoneal invasion of ovarian cancer and TF-targeted intraperitoneal anti-cancer treatment. We observed and propose a novel strategy for peritoneal implantation of ovarian cancer. The strategy includes the preinvasive growth of fibrin-anchored cancer cells along with neovascularization on the outer side of the intact peritoneal surface.
Keywords: extraperitoneal neovascularization; mesothelial barrier; ovarian cancer cell mass; peritoneal invasion; tissue factor.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



Similar articles
-
Ascites microenvironment conditions the peritoneal pre-metastatic niche to promote the implantation of ovarian tumor spheroids: Involvement of fibrinogen/fibrin and αV and α5β1 integrins.Exp Cell Res. 2024 Aug 1;441(1):114155. doi: 10.1016/j.yexcr.2024.114155. Epub 2024 Jul 16. Exp Cell Res. 2024. PMID: 39002689
-
Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer.Oncol Rep. 2003 Jan-Feb;10(1):89-95. Oncol Rep. 2003. PMID: 12469150
-
A novel mechanism of neovascularization in peritoneal dissemination via cancer-associated mesothelial cells affected by TGF-β derived from ovarian cancer.Oncol Rep. 2018 Jan;39(1):193-200. doi: 10.3892/or.2017.6104. Epub 2017 Nov 20. Oncol Rep. 2018. PMID: 29192324
-
Procancerogenic activity of senescent cells: A case of the peritoneal mesothelium.Ageing Res Rev. 2018 May;43:1-9. doi: 10.1016/j.arr.2018.01.002. Epub 2018 Jan 31. Ageing Res Rev. 2018. PMID: 29355719 Review.
-
[Intraperitoneal invasiveness of ovarian cancer from the cellular and molecular perspective].Ginekol Pol. 2015 Oct;86(10):782-6. doi: 10.17772/gp/58751. Ginekol Pol. 2015. PMID: 26677589 Review. Polish.
Cited by
-
The Detection of Plasma Soluble Podoplanin of Patients with Breast Cancer and Its Clinical Signification.Cancer Manag Res. 2020 Dec 23;12:13207-13214. doi: 10.2147/CMAR.S281785. eCollection 2020. Cancer Manag Res. 2020. PMID: 33380828 Free PMC article.
-
Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor.Molecules. 2020 Aug 19;25(17):3769. doi: 10.3390/molecules25173769. Molecules. 2020. PMID: 32824997 Free PMC article.
-
Rosa hybrida Petal Extract Exhibits Antitumor Effects by Abrogating Tumor Progression and Angiogenesis in Bladder Cancer Both In Vivo and In Vitro.Integr Cancer Ther. 2022 Jan-Dec;21:15347354221114337. doi: 10.1177/15347354221114337. Integr Cancer Ther. 2022. PMID: 35912937 Free PMC article.
-
Peritoneal dissemination of high-grade serous ovarian cancer: pivotal roles of chromosomal instability and epigenetic dynamics.J Gynecol Oncol. 2022 Sep;33(5):e83. doi: 10.3802/jgo.2022.33.e83. J Gynecol Oncol. 2022. PMID: 36032027 Free PMC article. Review.
References
-
- Partridge EE, Barnes MN. Epithelial ovarian cancer: prevention, diagnosis, and treatment. CA Cancer J Clin. 1999;49:297‐320. - PubMed
-
- Ozols RF, Bookman MA, Connolly DC, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19‐24. - PubMed
-
- Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastasis: biology and pathology. Gynecol Oncol. 2009;113:8143‐8148. - PubMed
-
- Matte I, Legault CM, Garde‐Granger P, et al. Mesothelial cells interact with tumor cells for the formation of ovarian cancer multicellular spheroids in peritoneal effusions. Clin Exp Metastasis. 2006;33:829‐852. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

