Arabinose Alters Both Local and Distal H-D Exchange Rates in the Escherichia coli AraC Transcriptional Regulator
- PMID: 31199144
- PMCID: PMC11747938
- DOI: 10.1021/acs.biochem.9b00389
Arabinose Alters Both Local and Distal H-D Exchange Rates in the Escherichia coli AraC Transcriptional Regulator
Abstract
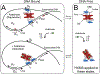
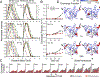
In the absence of arabinose, the dimeric Escherichia coli regulatory protein of the l-arabinose operon, AraC, represses expression by looping the DNA between distant half-sites. Binding of arabinose to the dimerization domains forces AraC to preferentially bind two adjacent DNA half-sites, which stimulates RNA polymerase transcription of the araBAD catabolism genes. Prior genetic and biochemical studies hypothesized that arabinose allosterically induces a helix-coil transition of a linker between the dimerization and DNA binding domains that switches the AraC conformation to an inducing state [Brown, M. J., and Schleif, R. F. (2019) Biochemistry, preceding paper in this issue (DOI: 10.1021/acs.biochem.9b00234)]. To test this hypothesis, hydrogen-deuterium exchange mass spectrometry was utilized to identify structural regions involved in the conformational activation of AraC by arabinose. Comparison of the hydrogen-deuterium exchange kinetics of individual dimeric dimerization domains and the full-length dimeric AraC protein in the presence and absence of arabinose reveals a prominent arabinose-induced destabilization of the amide hydrogen-bonded structure of linker residues (I167 and N168). This destabilization is demonstrated to result from an increased probability to form a helix capping motif at the C-terminal end of the dimerizing α-helix of the dimerization domain that preceeds the interdomain linker. These conformational changes could allow for quaternary repositioning of the DNA binding domains required for induction of the araBAD promoter through rotation of peptide backbone dihedral angles of just a couple of residues. Subtle changes in exchange rates are also visible around the arabinose binding pocket and in the DNA binding domain.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
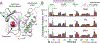


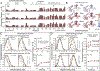
Similar articles
-
Helical Behavior of the Interdomain Linker of the Escherichia coli AraC Protein.Biochemistry. 2019 Jul 2;58(26):2867-2874. doi: 10.1021/acs.biochem.9b00234. Epub 2019 Jun 22. Biochemistry. 2019. PMID: 31199118
-
A new and unexpected domain-domain interaction in the AraC protein.Proteins. 2012 May;80(5):1465-75. doi: 10.1002/prot.24044. Epub 2012 Mar 1. Proteins. 2012. PMID: 22383259
-
A genetic and physical study of the interdomain linker of E. Coli AraC protein--a trans-subunit communication pathway.Proteins. 2016 Apr;84(4):448-60. doi: 10.1002/prot.24990. Epub 2016 Feb 5. Proteins. 2016. PMID: 26800223
-
AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action.FEMS Microbiol Rev. 2010 Sep;34(5):779-96. doi: 10.1111/j.1574-6976.2010.00226.x. Epub 2010 Apr 8. FEMS Microbiol Rev. 2010. PMID: 20491933 Review.
-
AraC protein: a love-hate relationship.Bioessays. 2003 Mar;25(3):274-82. doi: 10.1002/bies.10237. Bioessays. 2003. PMID: 12596232 Review.
Cited by
-
A Career's Work, the l-Arabinose Operon: How It Functions and How We Learned It.EcoSal Plus. 2022 Dec 15;10(1):eESP00122021. doi: 10.1128/ecosalplus.ESP-0012-2021. Epub 2021 Aug 18. EcoSal Plus. 2022. PMID: 36519894 Free PMC article. Review.
-
cAMP is an allosteric modulator of DNA-binding specificity in the cAMP receptor protein from Mycobacterium tuberculosis.J Biol Chem. 2021 Jan-Jun;296:100480. doi: 10.1016/j.jbc.2021.100480. Epub 2021 Feb 26. J Biol Chem. 2021. PMID: 33640453 Free PMC article.
-
Allosteric regulation within the highly interconnected structural scaffold of AraC/XylS homologs tolerates a wide range of amino acid changes.Proteins. 2022 Jan;90(1):186-199. doi: 10.1002/prot.26206. Epub 2021 Aug 16. Proteins. 2022. PMID: 34369028 Free PMC article.
-
H/D Exchange Characterization of Silent Coupling: Entropy-Enthalpy Compensation in Allostery.Biophys J. 2020 Jun 16;118(12):2966-2978. doi: 10.1016/j.bpj.2020.05.012. Epub 2020 May 20. Biophys J. 2020. PMID: 32479745 Free PMC article.
References
-
- Steffen D, and Schleif R (1977) In vitro construction of plasmids which result in overproduction of the protein product of the araC gene of Escherichia coli. Mol. Gen. Genet. 157, 341–344. - PubMed
-
- Sheppard DE, and Englesberg E (1967) Further evidence for positive control of the L-arabinose system by gene araC. J. Mol. Biol. 25, 443–454. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

