Guided Self-Help Works: Randomized Waitlist Controlled Trial of Pacifica, a Mobile App Integrating Cognitive Behavioral Therapy and Mindfulness for Stress, Anxiety, and Depression
- PMID: 31199319
- PMCID: PMC6592477
- DOI: 10.2196/12556
Guided Self-Help Works: Randomized Waitlist Controlled Trial of Pacifica, a Mobile App Integrating Cognitive Behavioral Therapy and Mindfulness for Stress, Anxiety, and Depression
Abstract
Background: Despite substantial improvements in technology and the increased demand for technology-enabled behavioral health tools among consumers, little progress has been made in easing the burden of mental illness. This may be because of the inherent challenges of conducting traditional clinical trials in a rapidly evolving technology landscape.
Objective: This study sought to validate the effectiveness of Pacifica, a popular commercially available app for the self-management of mild-to-moderate stress, anxiety, and depression.
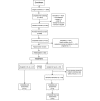
Methods: A total of 500 adults with mild-to-moderate anxiety or depression were recruited from in-app onboarding to participate in a randomized waitlist controlled trial of Pacifica. We conducted an all-virtual study, recruiting, screening, and randomizing participants through a Web-based participant portal. Study participants used the app for 1 month, with no level of use required, closely mimicking real-world app usage. Participants in the waitlist group were given access to the app after 1 month. Measurements included self-reported symptoms of stress, anxiety, depression, and self-efficacy. We performed an intent-to-treat analysis to examine the interactive effects of time and condition.
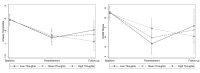
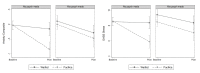
Results: We found significant interactions between time and group. Participants in the active condition demonstrated significantly greater decreases in depression, anxiety, and stress and increases in self-efficacy. Although we did not find a relationship between overall engagement with the app and symptom improvement, participants who completed relatively more thought record exercises sustained improvements in their symptoms through the 2-month follow-up to a greater degree than those who completed fewer. In addition, we found that participants who reported concomitantly taking psychiatric medications during the trial benefitted less from the app, as measured by the symptoms of anxiety and stress.
Conclusions: This study provides evidence that Pacifica, a popular commercially available self-help app, is effective in reducing self-reported symptoms of depression, anxiety, and stress, particularly among individuals who utilize thought records and are not taking psychiatric medication.
Trial registration: ClinicalTrials.gov NCT03333707; https://clinicaltrials.gov/ct2/show/NCT03333707 (Archived by WebCite at http://www.webcitation.org/78YE07ADB).
Keywords: anxiety; cognitive behavioral therapy; depression; mHealth; smartphone app; stress.
©Christine Moberg, Andrea Niles, Dale Beermann. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 08.06.2019.
Conflict of interest statement
Conflicts of Interest: At the time of this study, CM was the Head of Psychology, owned options in Pacifica Labs, Inc, and was receiving a salary from the company. DB was the Cofounder and CEO of Pacifica Labs, Inc, owned a large share of the company’s stock, and received a salary from the company.
Figures

Similar articles
-
Mobile Phone-Delivered Cognitive Behavioral Therapy for Insomnia: A Randomized Waitlist Controlled Trial.J Med Internet Res. 2017 Apr 11;19(4):e70. doi: 10.2196/jmir.6524. J Med Internet Res. 2017. PMID: 28400355 Free PMC article. Clinical Trial.
-
Smartphone Cognitive Behavioral Therapy as an Adjunct to Pharmacotherapy for Refractory Depression: Randomized Controlled Trial.J Med Internet Res. 2017 Nov 3;19(11):e373. doi: 10.2196/jmir.8602. J Med Internet Res. 2017. PMID: 29101095 Free PMC article. Clinical Trial.
-
A Web-Based Cognitive Behavior Therapy Intervention to Improve Social and Occupational Functioning in Adults With Type 2 Diabetes (The SpringboarD Trial): Randomized Controlled Trial.J Med Internet Res. 2019 May 21;21(5):e12246. doi: 10.2196/12246. J Med Internet Res. 2019. PMID: 31115345 Free PMC article. Clinical Trial.
-
Effects of Mindfulness Exercise Guided by a Smartphone App on Negative Emotions and Stress in Non-Clinical Populations: A Systematic Review and Meta-Analysis.Front Public Health. 2022 Jan 25;9:773296. doi: 10.3389/fpubh.2021.773296. eCollection 2021. Front Public Health. 2022. PMID: 35155341 Free PMC article.
-
The effects of mobile apps on stress, anxiety, and depression: overview of systematic reviews.Int J Technol Assess Health Care. 2020 Dec 14;37:e4. doi: 10.1017/S0266462320002093. Int J Technol Assess Health Care. 2020. PMID: 33314997 Review.
Cited by
-
A randomized controlled trial of a 14-day mindfulness ecological momentary intervention (MEMI) for generalized anxiety disorder.Eur Psychiatry. 2023 Jan 16;66(1):e12. doi: 10.1192/j.eurpsy.2023.2. Eur Psychiatry. 2023. PMID: 36645098 Free PMC article. Clinical Trial.
-
Healthy Dwelling: Design of Biophilic Interior Environments Fostering Self-Care Practices for People Living with Migraines, Chronic Pain, and Depression.Int J Environ Res Public Health. 2022 Feb 16;19(4):2248. doi: 10.3390/ijerph19042248. Int J Environ Res Public Health. 2022. PMID: 35206441 Free PMC article.
-
The efficacy of mindfulness apps on symptoms of depression and anxiety: An updated meta-analysis of randomized controlled trials.Clin Psychol Rev. 2024 Feb;107:102370. doi: 10.1016/j.cpr.2023.102370. Epub 2023 Dec 3. Clin Psychol Rev. 2024. PMID: 38056219 Free PMC article. Review.
-
Engagement Strategies to Improve Adherence and Retention in Web-Based Mindfulness Programs: Systematic Review.J Med Internet Res. 2022 Jan 12;24(1):e30026. doi: 10.2196/30026. J Med Internet Res. 2022. PMID: 35019851 Free PMC article.
-
Characteristics of Mobile Health Platforms for Depression and Anxiety: Content Analysis Through a Systematic Review of the Literature and Systematic Search of Two App Stores.J Med Internet Res. 2022 Feb 4;24(2):e27388. doi: 10.2196/27388. J Med Internet Res. 2022. PMID: 35119370 Free PMC article.
References
-
- National Comorbidity Survey (NCS) 2017. [2019-05-20]. https://www.hcp.med.harvard.edu/ncs/index.php .
-
- Key Substance Use and Mental Health Indicators in the United States: Results from the 2016 National Survey on Drug Use and Health. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2017.
-
- Beck J. Cognitive Behavior Therapy, Second Edition. New York, NY: Guilford Press; 2011. p. 9781609185046.
-
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, O'Reardon JP, Lovett ML, Gladis MM, Brown LL, Gallop R. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005 Apr;62(4):409–16. doi: 10.1001/archpsyc.62.4.409.62/4/409 - DOI - PubMed

