Severe acute respiratory syndrome coronavirus spike protein counteracts BST2-mediated restriction of virus-like particle release
- PMID: 31199522
- PMCID: PMC7166632
- DOI: 10.1002/jmv.25518
Severe acute respiratory syndrome coronavirus spike protein counteracts BST2-mediated restriction of virus-like particle release
Abstract
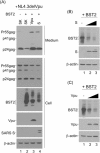
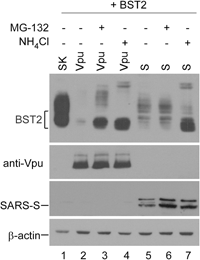
BST2/tetherin, an interferon-inducible antiviral factor, can block the cellular release of various enveloped viruses. We previously reported that human coronavirus 229E (HCoV-229E) infection can alleviate the BST2 tethering of HIV-1 virions by downregulating cell surface BST2, suggesting that coronaviruses are capable of encoding anti-BST2 factors. Here we report our new finding that severe acute respiratory syndrome coronavirus (SARS-CoV) spike (S) glycoprotein, similar to Vpu, is capable of antagonizing the BST2 tethering of SARS-CoV, HCoV-229E, and HIV-1 virus-like particles via BST2 downregulation. However, unlike Vpu (which downmodulates BST2 by means of proteasomal and lysosomal degradation pathways), BST2 downregulation is apparently mediated by SARS-CoV S through the lysosomal degradation pathway only. We found that SARS-CoV S colocalized with both BST2 and reduced cell surface BST2, suggesting an association between SARS-CoV S and BST2 that targets the lysosomal degradation pathway. According to one recent report, SARS-CoV ORF7a antagonizes BST2 by interfering with BST2 glycosylation1 . Our data provide support for the proposal that SARS-CoV and other enveloped viruses are capable of evolving supplementary anti-BST2 factors in a manner that requires virus replication. Further experiments are required to determine whether the BST2-mediated restriction of authentic SARS-CoV virions is alleviated by the SARS-CoV spike protein.
Keywords: SARS coronavirus; coronavirus; human immunodeficiency virus; immune responses; innate immunity; virus classification.
© 2019 Wiley Periodicals, Inc.
Figures




References
-
- Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Bst‐2/HM1.24 is a raft‐associated apical membrane protein with an unusual topology. Traffic. 2003;4(10):694‐709. - PubMed
-
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV‐1 Vpu. Nature. 2008;451(7177):425‐430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

