The S52F FOXF1 Mutation Inhibits STAT3 Signaling and Causes Alveolar Capillary Dysplasia
- PMID: 31199666
- PMCID: PMC6794119
- DOI: 10.1164/rccm.201810-1897OC
The S52F FOXF1 Mutation Inhibits STAT3 Signaling and Causes Alveolar Capillary Dysplasia
Abstract
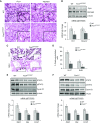
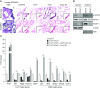
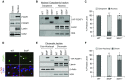
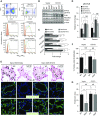
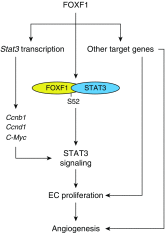
Rationale: Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is a lethal congenital disorder causing respiratory failure and pulmonary hypertension shortly after birth. There are no effective treatments for ACDMPV other than lung transplant, and new therapeutic approaches are urgently needed. Although ACDMPV is linked to mutations in the FOXF1 gene, molecular mechanisms through which FOXF1 mutations cause ACDMPV are unknown.Objectives: To identify molecular mechanisms by which S52F FOXF1 mutations cause ACDMPV.Methods: We generated a clinically relevant mouse model of ACDMPV by introducing the S52F FOXF1 mutation into the mouse Foxf1 gene locus using CRISPR/Cas9 technology. Immunohistochemistry, whole-lung imaging, and biochemical methods were used to examine vasculature in Foxf1WT/S52F lungs and identify molecular mechanisms regulated by FOXF1.Measurements and Main Results: FOXF1 mutations were identified in 28 subjects with ACDMPV. Foxf1WT/S52F knock-in mice recapitulated histopathologic findings in ACDMPV infants. The S52F FOXF1 mutation disrupted STAT3-FOXF1 protein-protein interactions and inhibited transcription of Stat3, a critical transcriptional regulator of angiogenesis. STAT3 signaling and endothelial proliferation were reduced in Foxf1WT/S52F mice and human ACDMPV lungs. S52F FOXF1 mutant protein did not bind chromatin and was transcriptionally inactive. Furthermore, we have developed a novel formulation of highly efficient nanoparticles and demonstrated that nanoparticle delivery of STAT3 cDNA into the neonatal circulation restored endothelial proliferation and stimulated lung angiogenesis in Foxf1WT/S52F mice.Conclusions: FOXF1 acts through STAT3 to stimulate neonatal lung angiogenesis. Nanoparticle delivery of STAT3 is a promising strategy to treat ACDMPV associated with decreased STAT3 signaling.
Keywords: ACDMPV; FOXF1 transcription factor; STAT3; alveolar capillary dysplasia with misalignment of pulmonary veins; neonatal pulmonary angiogenesis.
Figures


Comment in
-
A Step toward Treating a Lethal Neonatal Lung Disease. STAT3 and Alveolar Capillary Dysplasia.Am J Respir Crit Care Med. 2019 Oct 15;200(8):961-962. doi: 10.1164/rccm.201906-1102ED. Am J Respir Crit Care Med. 2019. PMID: 31343895 Free PMC article. No abstract available.
References
-
- Stankiewicz P, Sen P, Bhatt SS, Storer M, Xia Z, Bejjani BA, et al. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations Am J Hum Genet 200984780–791.[Published erratum appears in Am J Hum Genet 85:537.] - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

