SLAMF6 clustering is required to augment T cell activation
- PMID: 31199820
- PMCID: PMC6568412
- DOI: 10.1371/journal.pone.0218109
SLAMF6 clustering is required to augment T cell activation
Abstract
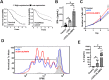
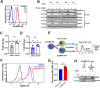
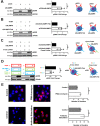
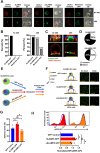
The signaling lymphocytic activation molecule (SLAM) family is comprised of nine distinct receptors that are expressed exclusively on hematopoietic cells. Most of these transmembrane receptors are homotypic by nature and downstream signaling occurs when cells that express the same SLAM receptor interact. Previous studies have determined that anti-SLAMF6 antibodies can have a therapeutic effect in autoimmunity and cancer. However, little is known about the role of SLAMF6 in the adaptive immune responses and in order to utilize SLAMF6 interventional approaches, a better understanding of the biology of this receptor in T cell is warranted. Accordingly, the objective of our study was to investigate both functionally and structurally the role of SLAMF6 in T cell receptor (TCR) mediated responses. Biochemical and genetic experiments revealed that SLAMF6 was required for productive TCR downstream signaling. Interestingly, SLAMF6 ectodomain was required for its function, but not for its recruitment to the immunological synapse. Flow-cytometry analysis demonstrated that tyrosine 308 of the tail of SLAMF6 was crucial for its ability to enhance T cell function. Imaging studies revealed that SLAMF6 clustering, specifically with the TCR, resulted in dramatic increase in downstream signaling. Mechanistically, we showed that SLAMF6 enhanced T cell function by increasing T cell adhesiveness through activation of the small GTPase Rap1. Taken together SLAMF6 is an important regulator of T cell activation where both its ectodomain and its endodomain contribute differentially to T cell functions. Additional studies are underway to better evaluate the role of anti-SLAMF6 approaches in specific human diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
SLAMF6 compartmentalization enhances T cell functions.Life Sci Alliance. 2022 Dec 8;6(2):e202201533. doi: 10.26508/lsa.202201533. Print 2023 Feb. Life Sci Alliance. 2022. PMID: 36622343 Free PMC article.
-
Soluble SLAMF6 Receptor Induces Strong CD8+ T-cell Effector Function and Improves Anti-Melanoma Activity In Vivo.Cancer Immunol Res. 2018 Feb;6(2):127-138. doi: 10.1158/2326-6066.CIR-17-0383. Epub 2018 Jan 5. Cancer Immunol Res. 2018. PMID: 29305520
-
Slamf6 negatively regulates autoimmunity.Clin Immunol. 2016 Dec;173:19-26. doi: 10.1016/j.clim.2016.06.009. Epub 2016 Jun 29. Clin Immunol. 2016. PMID: 27368806 Free PMC article.
-
The Role of Adaptor Proteins in the Biology of Natural Killer T (NKT) Cells.Front Immunol. 2019 Jun 25;10:1449. doi: 10.3389/fimmu.2019.01449. eCollection 2019. Front Immunol. 2019. PMID: 31293596 Free PMC article. Review.
-
The SLAM family receptors: Potential therapeutic targets for inflammatory and autoimmune diseases.Autoimmun Rev. 2018 Jul;17(7):674-682. doi: 10.1016/j.autrev.2018.01.018. Epub 2018 May 3. Autoimmun Rev. 2018. PMID: 29729453 Free PMC article. Review.
Cited by
-
Clinical and immunological relevance of SLAMF6 expression in the tumor microenvironment of breast cancer and melanoma.Sci Rep. 2024 Jan 29;14(1):2394. doi: 10.1038/s41598-023-50062-y. Sci Rep. 2024. PMID: 38287061 Free PMC article.
-
SLAMF6 enables efficient attachment, synapse formation, and killing of HIV-1-infected CD4+ T cells by virus-specific CD8+ T cells.bioRxiv [Preprint]. 2025 Jan 22:2025.01.20.633914. doi: 10.1101/2025.01.20.633914. bioRxiv. 2025. PMID: 39896504 Free PMC article. Preprint.
-
The Role of Immune Checkpoint Receptors in Regulating Immune Reactivity in Lupus.Cells. 2019 Oct 8;8(10):1213. doi: 10.3390/cells8101213. Cells. 2019. PMID: 31597242 Free PMC article. Review.
-
Causal Characteristics of Immune Cells Associated with Aortic Dissection: A Mendelian Randomisation Analysis.Eur Cardiol. 2025 Apr 1;20:e07. doi: 10.15420/ecr.2024.44. eCollection 2025. Eur Cardiol. 2025. PMID: 40201453 Free PMC article.
-
Chimeric Antigen Receptor (CAR) T Cells Releasing Soluble SLAMF6 Isoform 2 Gain Superior Anti-Cancer Cell Functionality in an Auto-Stimulatory Fashion.Cells. 2025 Jun 14;14(12):901. doi: 10.3390/cells14120901. Cells. 2025. PMID: 40558528 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

