Kaiso-induced intestinal inflammation is preceded by diminished E-cadherin expression and intestinal integrity
- PMID: 31199830
- PMCID: PMC6568390
- DOI: 10.1371/journal.pone.0217220
Kaiso-induced intestinal inflammation is preceded by diminished E-cadherin expression and intestinal integrity
Abstract
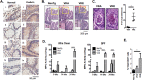
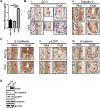
Chronic intestinal inflammation contributes to pathologies such as inflammatory bowel disease (IBD) and colon cancer. While the precise etiology remains controversial, IBD is believed to manifest as a result of various factors. We previously reported that intestinal-specific overexpression of the transcription factor Kaiso results in an intestinal inflammatory response; however, the cause of this inflammation is unknown. To elucidate the underlying mechanism(s) of the Kaiso-mediated intestinal inflammatory phenotype, we evaluated two independent transgenic mouse lines that express varying levels of Kaiso (KaisoTg). Histological analyses of KaisoTg mice revealed intestinal damage including thickening of the mucosa, intestinal "lesions" and crypt abscesses, which are reminiscent of IBD pathology. Additionally, higher Kaiso levels induced intestinal neutrophilia as early as 12 weeks, which worsened as the mice aged. Notably, the Kaiso-induced intestinal inflammation correlated with a leaky intestinal barrier and mis-regulation of E-cadherin expression and localization. Interestingly, Kaiso overexpression resulted in reduced proliferation but enhanced migration of intestinal epithelial cells prior to the onset of inflammation. Collectively, these data suggest that Kaiso plays a role in regulating intestinal epithelial cell integrity and function, dysregulation of which contributes to a chronic inflammatory phenotype as mice age.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Kaiso differentially regulates components of the Notch signaling pathway in intestinal cells.Cell Commun Signal. 2017 Jun 21;15(1):24. doi: 10.1186/s12964-017-0178-x. Cell Commun Signal. 2017. PMID: 28637464 Free PMC article.
-
A new role for reticulon-4B/NOGO-B in the intestinal epithelial barrier function and inflammatory bowel disease.Am J Physiol Gastrointest Liver Physiol. 2015 Jun 15;308(12):G981-93. doi: 10.1152/ajpgi.00309.2014. Epub 2015 Apr 23. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25907690
-
MicroRNA 301A Promotes Intestinal Inflammation and Colitis-Associated Cancer Development by Inhibiting BTG1.Gastroenterology. 2017 May;152(6):1434-1448.e15. doi: 10.1053/j.gastro.2017.01.049. Epub 2017 Feb 11. Gastroenterology. 2017. PMID: 28193514
-
Dysregulation of Intestinal Epithelial Cell RIPK Pathways Promotes Chronic Inflammation in the IBD Gut.Front Immunol. 2019 May 20;10:1094. doi: 10.3389/fimmu.2019.01094. eCollection 2019. Front Immunol. 2019. PMID: 31164887 Free PMC article. Review.
Cited by
-
Decellularized small intestine scaffolds: a potential xenograft for restoration of intestinal perforation.Tissue Barriers. 2024 Oct;12(4):2290940. doi: 10.1080/21688370.2023.2290940. Epub 2023 Dec 5. Tissue Barriers. 2024. PMID: 38053224 Free PMC article.
-
Bacteroides fragilis Toxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 by the E-Cadherin/β-Catenin/NF-κB Dependent Pathway.Biomedicines. 2022 Mar 31;10(4):827. doi: 10.3390/biomedicines10040827. Biomedicines. 2022. PMID: 35453577 Free PMC article.
-
Inhibitors of DNA Methylation.Adv Exp Med Biol. 2022;1389:471-513. doi: 10.1007/978-3-031-11454-0_17. Adv Exp Med Biol. 2022. PMID: 36350520 Review.
-
Adherens junction proteins on the move-From the membrane to the nucleus in intestinal diseases.Front Cell Dev Biol. 2022 Oct 5;10:998373. doi: 10.3389/fcell.2022.998373. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36274850 Free PMC article. Review.
-
Kaiso phosphorylation at threonine 606 leads to its accumulation in the cytoplasm, reducing its transcriptional repression of the tumour suppressor CDH1.Mol Oncol. 2022 Sep;16(17):3192-3209. doi: 10.1002/1878-0261.13292. Epub 2022 Jul 28. Mol Oncol. 2022. PMID: 35851744 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

