ATP6V0d2 controls Leishmania parasitophorous vacuole biogenesis via cholesterol homeostasis
- PMID: 31199856
- PMCID: PMC6594656
- DOI: 10.1371/journal.ppat.1007834
ATP6V0d2 controls Leishmania parasitophorous vacuole biogenesis via cholesterol homeostasis
Abstract
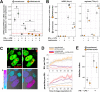
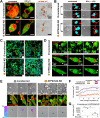
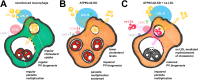
V-ATPases are part of the membrane components of pathogen-containing vacuoles, although their function in intracellular infection remains elusive. In addition to organelle acidification, V-ATPases are alternatively implicated in membrane fusion and anti-inflammatory functions controlled by ATP6V0d2, the d subunit variant of the V-ATPase complex. Therefore, we evaluated the role of ATP6V0d2 in the biogenesis of pathogen-containing vacuoles using ATP6V0d2 knock-down macrophages infected with the protozoan parasite Leishmania amazonensis. These parasites survive within IFNγ/LPS-activated inflammatory macrophages, multiplying in large/fusogenic parasitophorous vacuoles (PVs) and inducing ATP6V0d2 upregulation. ATP6V0d2 knock-down decreased macrophage cholesterol levels and inhibited PV enlargement without interfering with parasite multiplication. However, parasites required ATP6V0d2 to resist the influx of oxidized low-density lipoprotein (ox-LDL)-derived cholesterol, which restored PV enlargement in ATP6V0d2 knock-down macrophages by replenishing macrophage cholesterol pools. Thus, we reveal parasite-mediated subversion of host V-ATPase function toward cholesterol retention, which is required for establishing an inflammation-resistant intracellular parasite niche.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Changes to cholesterol trafficking in macrophages by Leishmania parasites infection.Microbiologyopen. 2017 Aug;6(4):e00469. doi: 10.1002/mbo3.469. Epub 2017 Mar 27. Microbiologyopen. 2017. PMID: 28349644 Free PMC article.
-
Leishmania infection-induced multinucleated giant cell formation via upregulation of ATP6V0D2 expression.Front Cell Infect Microbiol. 2022 Sep 23;12:953785. doi: 10.3389/fcimb.2022.953785. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36211967 Free PMC article.
-
Control of parasitophorous vacuole expansion by LYST/Beige restricts the intracellular growth of Leishmania amazonensis.PLoS Pathog. 2008 Oct;4(10):e1000179. doi: 10.1371/journal.ppat.1000179. Epub 2008 Oct 17. PLoS Pathog. 2008. PMID: 18927622 Free PMC article.
-
The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages.Trends Microbiol. 1998 Oct;6(10):392-401. doi: 10.1016/s0966-842x(98)01324-9. Trends Microbiol. 1998. PMID: 9807783 Review.
-
The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival.Front Cell Dev Biol. 2020 Jun 10;8:396. doi: 10.3389/fcell.2020.00396. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32587854 Free PMC article. Review.
Cited by
-
Upregulation of ATP6V0D2 benefits intracellular survival of Leishmania donovani in erythrocytes-engulfing macrophages.Front Cell Infect Microbiol. 2024 Jan 31;14:1332381. doi: 10.3389/fcimb.2024.1332381. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38357442 Free PMC article.
-
VAMP3 and VAMP8 Regulate the Development and Functionality of Parasitophorous Vacuoles Housing Leishmania amazonensis.Infect Immun. 2022 Mar 17;90(3):e0018321. doi: 10.1128/IAI.00183-21. Epub 2022 Feb 7. Infect Immun. 2022. PMID: 35130453 Free PMC article.
-
Fatty Acid Composition and Metabolism in Leishmania Parasite Species: Potential Biomarkers or Drug Targets for Leishmaniasis?Int J Mol Sci. 2023 Feb 28;24(5):4702. doi: 10.3390/ijms24054702. Int J Mol Sci. 2023. PMID: 36902138 Free PMC article. Review.
-
CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate.J Exp Med. 2022 Jun 6;219(6):e20211314. doi: 10.1084/jem.20211314. Epub 2022 Apr 19. J Exp Med. 2022. PMID: 35438721 Free PMC article. Review.
-
The Paradox of a Phagosomal Lifestyle: How Innate Host Cell-Leishmania amazonensis Interactions Lead to a Progressive Chronic Disease.Front Immunol. 2021 Sep 7;12:728848. doi: 10.3389/fimmu.2021.728848. eCollection 2021. Front Immunol. 2021. PMID: 34557194 Free PMC article. Review.
References
-
- Lukacs GL, Rotstein OD, Grinstein S. Phagosomal acidification is mediated by a vacuolar-type H(+)-ATPase in murine macrophages. J Biol Chem. 1990;265(34):21099–107. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

