A scorpion venom peptide derivative BmKn‒22 with potent antibiofilm activity against Pseudomonas aeruginosa
- PMID: 31199859
- PMCID: PMC6568410
- DOI: 10.1371/journal.pone.0218479
A scorpion venom peptide derivative BmKn‒22 with potent antibiofilm activity against Pseudomonas aeruginosa
Abstract
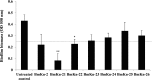
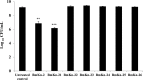
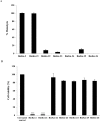
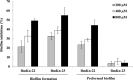
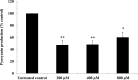
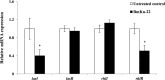
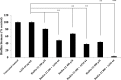
Pseudomonas aeruginosa is a leading cause of nosocomial and serious life-threatening infections and infections caused by this bacterium continue to pose a major medical challenge worldwide. The ability of P. aeruginosa to produce multiple virulence factors and in particular to form biofilms makes this bacterium resistant to all known antibiotics. As a consequence, standard antibiotic therapy are increasingly become ineffective to clear such infections associated with biofilms. In search for novel effective agents to combat P. aeruginosa biofilm infections, a series of the BmKn‒2 scorpion venom peptide and its truncated derivatives were synthesized and their antibiofilm activities assessed. Among the peptides tested, BmKn‒22 peptide, which was a modified peptide of the parental BmKn‒2 scorpion venom peptide, clearly demonstrated the most potential inhibitory activity against P. aeruginosa biofilms without affecting the bacterial growth. This peptide was not only capable of inhibiting the formation of P. aeruginosa biofilms, but also disrupting the established biofilms of P. aeruginosa. Additionally, BmKn‒22 peptide was able to inhibit the production of key virulence factor pyocyanin of P. aeruginosa. Our results also showed that BmKn‒22 peptide significantly reduced lasI and rhlR expression, and suggested that BmKn‒22 peptide-mediated inhibition of P. aeruginosa biofilms and virulence factors was achieved through the components of quorum-sensing systems. Combination of BmKn‒22 peptide with azithromycin resulted in a remarkable reduction P. aeruginosa biofilms. Since this peptide exhibited low toxicity to mammalian cells, all our results therefore indicate that the BmKn‒22 peptide is a promising antibiofilm agent against P. aeruginosa and warrant further development of this peptide as a novel therapeutic for treatment of P. aeruginosa‒associated biofilm infections.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Two novel synthetic peptides inhibit quorum sensing-dependent biofilm formation and some virulence factors in Pseudomonas aeruginosa PAO1.J Microbiol. 2019 Jul;57(7):618-625. doi: 10.1007/s12275-019-8548-2. Epub 2019 Jun 27. J Microbiol. 2019. PMID: 31054133
-
Baicalin inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances Pseudomonas aeruginosa clearance in a mouse peritoneal implant infection model.PLoS One. 2017 Apr 28;12(4):e0176883. doi: 10.1371/journal.pone.0176883. eCollection 2017. PLoS One. 2017. PMID: 28453568 Free PMC article.
-
Lutein: A potential antibiofilm and antiquorum sensing molecule from green microalga Chlorella pyrenoidosa.Microb Pathog. 2019 Oct;135:103658. doi: 10.1016/j.micpath.2019.103658. Epub 2019 Aug 6. Microb Pathog. 2019. PMID: 31398531
-
Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors.Future Microbiol. 2013 Jul;8(7):901-21. doi: 10.2217/fmb.13.57. Future Microbiol. 2013. PMID: 23841636 Review.
-
Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies.J Biotechnol. 2014 Dec 10;191:121-30. doi: 10.1016/j.jbiotec.2014.09.003. Epub 2014 Sep 18. J Biotechnol. 2014. PMID: 25240440 Review.
Cited by
-
Antimicrobial Potential of Scorpion-Venom-Derived Peptides.Molecules. 2024 Oct 27;29(21):5080. doi: 10.3390/molecules29215080. Molecules. 2024. PMID: 39519721 Free PMC article. Review.
-
Anti-Biofilm and Anti-Inflammatory Properties of the Truncated Analogs of the Scorpion Venom-Derived Peptide IsCT against Pseudomonas aeruginosa.Antibiotics (Basel). 2024 Aug 16;13(8):775. doi: 10.3390/antibiotics13080775. Antibiotics (Basel). 2024. PMID: 39200075 Free PMC article.
-
The investigation of antibacterial properties of peptides and protein hydrolysates derived from serum of Asian water monitor (Varanus salvator).PLoS One. 2023 Oct 18;18(10):e0292947. doi: 10.1371/journal.pone.0292947. eCollection 2023. PLoS One. 2023. PMID: 37851665 Free PMC article.
-
Antimicrobial Peptides Derived From Insects Offer a Novel Therapeutic Option to Combat Biofilm: A Review.Front Microbiol. 2021 Jun 10;12:661195. doi: 10.3389/fmicb.2021.661195. eCollection 2021. Front Microbiol. 2021. PMID: 34248873 Free PMC article. Review.
-
Venom biotechnology: casting light on nature's deadliest weapons using synthetic biology.Front Bioeng Biotechnol. 2023 May 3;11:1166601. doi: 10.3389/fbioe.2023.1166601. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37207126 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

