Viral Capsid Trafficking along Treadmilling Tubulin Filaments in Bacteria
- PMID: 31199917
- PMCID: PMC7301877
- DOI: 10.1016/j.cell.2019.05.032
Viral Capsid Trafficking along Treadmilling Tubulin Filaments in Bacteria
Abstract
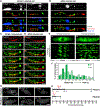
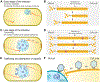
Cargo trafficking along microtubules is exploited by eukaryotic viruses, but no such examples have been reported in bacteria. Several large Pseudomonas phages assemble a dynamic, tubulin-based (PhuZ) spindle that centers replicating phage DNA sequestered within a nucleus-like structure. Here, we show that capsids assemble on the membrane and then move rapidly along PhuZ filaments toward the phage nucleus for DNA packaging. The spindle rotates the phage nucleus, distributing capsids around its surface. PhuZ filaments treadmill toward the nucleus at a constant rate similar to the rate of capsid movement and the linear velocity of nucleus rotation. Capsids become trapped along mutant static PhuZ filaments that are defective in GTP hydrolysis. Our results suggest a transport and distribution mechanism in which capsids attached to the sides of filaments are trafficked to the nucleus by PhuZ polymerization at the poles, demonstrating that the phage cytoskeleton evolved cargo-trafficking capabilities in bacteria.
Keywords: PhuZ; Pseudomonas phage; capsid distribution; capsid trafficking; giant phage; nuclear rotation; phage nucleus; treadmilling.
Copyright © 2019. Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures




Similar articles
-
Intracellular Organization by Jumbo Bacteriophages.J Bacteriol. 2020 Dec 18;203(2):e00362-20. doi: 10.1128/JB.00362-20. Print 2020 Dec 18. J Bacteriol. 2020. PMID: 32868402 Free PMC article. Review.
-
The Phage Nucleus and Tubulin Spindle Are Conserved among Large Pseudomonas Phages.Cell Rep. 2017 Aug 15;20(7):1563-1571. doi: 10.1016/j.celrep.2017.07.064. Cell Rep. 2017. PMID: 28813669 Free PMC article.
-
A cytoskeletal vortex drives phage nucleus rotation during jumbo phage replication in E. coli.Cell Rep. 2022 Aug 16;40(7):111179. doi: 10.1016/j.celrep.2022.111179. Cell Rep. 2022. PMID: 35977483 Free PMC article.
-
A phage tubulin assembles dynamic filaments by an atypical mechanism to center viral DNA within the host cell.Cell. 2012 Jun 22;149(7):1488-99. doi: 10.1016/j.cell.2012.04.034. Cell. 2012. PMID: 22726436 Free PMC article.
-
The Phage Nucleus and PhuZ Spindle: Defining Features of the Subcellular Organization and Speciation of Nucleus-Forming Jumbo Phages.Front Microbiol. 2021 Jul 13;12:641317. doi: 10.3389/fmicb.2021.641317. eCollection 2021. Front Microbiol. 2021. PMID: 34326818 Free PMC article. Review.
Cited by
-
Eukaryogenesis: The Rise of an Emergent Superorganism.Front Microbiol. 2022 May 11;13:858064. doi: 10.3389/fmicb.2022.858064. eCollection 2022. Front Microbiol. 2022. PMID: 35633668 Free PMC article.
-
Practical Approaches for Cryo-FIB Milling and Applications for Cellular Cryo-Electron Tomography.Methods Mol Biol. 2021;2215:49-82. doi: 10.1007/978-1-0716-0966-8_3. Methods Mol Biol. 2021. PMID: 33367999 Free PMC article.
-
Intracellular Organization by Jumbo Bacteriophages.J Bacteriol. 2020 Dec 18;203(2):e00362-20. doi: 10.1128/JB.00362-20. Print 2020 Dec 18. J Bacteriol. 2020. PMID: 32868402 Free PMC article. Review.
-
Subcellular organization of viral particles during maturation of nucleus-forming jumbo phage.Sci Adv. 2022 May 6;8(18):eabj9670. doi: 10.1126/sciadv.abj9670. Epub 2022 May 4. Sci Adv. 2022. PMID: 35507660 Free PMC article.
-
Bacterial cytological profiling reveals interactions between jumbo phage φKZ infection and cell wall active antibiotics in Pseudomonas aeruginosa.PLoS One. 2023 Jul 7;18(7):e0280070. doi: 10.1371/journal.pone.0280070. eCollection 2023. PLoS One. 2023. PMID: 37418366 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

