The role of the neuropeptide PEN receptor, GPR83, in the reward pathway: Relationship to sex-differences
- PMID: 31199956
- PMCID: PMC6677605
- DOI: 10.1016/j.neuropharm.2019.107666
The role of the neuropeptide PEN receptor, GPR83, in the reward pathway: Relationship to sex-differences
Abstract
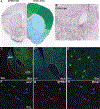
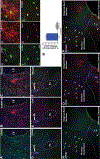
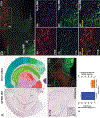
GPR83, the receptor for the neuropeptide PEN, exhibits high expression in the nucleus accumbens of the human and rodent brain, suggesting that it plays a role in modulating the mesolimbic reward pathway. However, the cell-type specific expression of GPR83, its functional impact in the reward pathway, and in drug reward-learning has not been fully explored. Using GPR83/eGFP mice, we show high GPR83 expression on cholinergic interneurons in the nucleus accumbens and moderate expression on ventral tegmental area dopamine neurons. In GPR83 knockout mice, baseline dopamine release in the nucleus accumbens is enhanced which disrupts the ratio of tonic vs phasic release. Additionally, GPR83 knockout leads to changes in the expression of dopamine-related genes. Using the morphine conditioned place preference model, we identify sex differences in morphine reward-learning, show that GPR83 is upregulated in the nucleus accumbens following morphine conditioned place preference, and show that shRNA-mediated knockdown of GPR83 in the nucleus accumbens leads to attenuation morphine reward. Together, these findings detect GPR83 expression in the reward-pathway, and show its involvement in dopamine release and morphine reward-learning.
Keywords: Cholinergic interneurons; Dopamine; GIR; Morphine; Voltammetry; proSAAS.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing.Cell Rep. 2012 Jul 26;2(1):33-41. doi: 10.1016/j.celrep.2012.05.011. Epub 2012 Jul 11. Cell Rep. 2012. PMID: 22840394 Free PMC article.
-
Optogenetically-induced tonic dopamine release from VTA-nucleus accumbens projections inhibits reward consummatory behaviors.Neuroscience. 2016 Oct 1;333:54-64. doi: 10.1016/j.neuroscience.2016.07.006. Epub 2016 Jul 13. Neuroscience. 2016. PMID: 27421228 Free PMC article.
-
Relationship between the role of muscarinic M3 receptors in morphine-induced conditioned place preference and the mesolimbic dopamine system.Neurosci Lett. 2022 Aug 24;786:136774. doi: 10.1016/j.neulet.2022.136774. Epub 2022 Jul 7. Neurosci Lett. 2022. PMID: 35809878
-
Neuropeptide PEN and Its Receptor GPR83: Distribution, Signaling, and Regulation.ACS Chem Neurosci. 2019 Apr 17;10(4):1884-1891. doi: 10.1021/acschemneuro.8b00559. Epub 2019 Feb 21. ACS Chem Neurosci. 2019. PMID: 30726666 Free PMC article. Review.
-
Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction.Eur J Pharmacol. 2003 Nov 7;480(1-3):117-23. doi: 10.1016/j.ejphar.2003.08.099. Eur J Pharmacol. 2003. PMID: 14623355 Review.
Cited by
-
Nanomolar range of FAM237B can activate receptor GPR83.Amino Acids. 2023 Nov;55(11):1557-1562. doi: 10.1007/s00726-023-03328-8. Epub 2023 Sep 9. Amino Acids. 2023. PMID: 37689599
-
A small molecule ligand for the novel pain target, GPR171, produces minimal reward in mice.Pharmacol Biochem Behav. 2023 Mar;224:173543. doi: 10.1016/j.pbb.2023.173543. Epub 2023 Mar 17. Pharmacol Biochem Behav. 2023. PMID: 36933620 Free PMC article.
-
PEN Receptor GPR83 in Anxiety-Like Behaviors: Differential Regulation in Global vs Amygdalar Knockdown.Front Neurosci. 2021 Aug 26;15:675769. doi: 10.3389/fnins.2021.675769. eCollection 2021. Front Neurosci. 2021. PMID: 34512237 Free PMC article.
-
GPR83 engages endogenous peptides from two distinct precursors to elicit differential signaling.Mol Pharmacol. 2022 May 23;102(1):29-38. doi: 10.1124/molpharm.122.000487. Mol Pharmacol. 2022. PMID: 35605991 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DP1 DA048931/DA/NIDA NIH HHS/United States
- R37 DA008863/DA/NIDA NIH HHS/United States
- R01 NS026880/NS/NINDS NIH HHS/United States
- R00 DA042111/DA/NIDA NIH HHS/United States
- R01 DA008863/DA/NIDA NIH HHS/United States
- T32 DA041349/DA/NIDA NIH HHS/United States
- R56 MH115409/MH/NIMH NIH HHS/United States
- K99 DA042111/DA/NIDA NIH HHS/United States
- R01 MH092306/MH/NIMH NIH HHS/United States
- R21 MH112081/MH/NIMH NIH HHS/United States
- R01 MH120637/MH/NIMH NIH HHS/United States
- R01 AA022445/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

