Current Aspects of siRNA Bioconjugate for In Vitro and In Vivo Delivery
- PMID: 31200490
- PMCID: PMC6631009
- DOI: 10.3390/molecules24122211
Current Aspects of siRNA Bioconjugate for In Vitro and In Vivo Delivery
Abstract
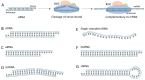
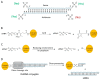
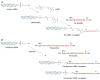
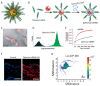
Studies on siRNA delivery have seen intense growth in the past decades since siRNA has emerged as a new class of gene therapeutics for the treatment of various diseases. siRNA bioconjugate, as one of the major delivery strategies, offers the potential to enhance and broaden pharmacological properties of siRNA, while minimizing the heterogeneity and stability-correlated toxicology. This review summarizes the recent developments of siRNA bioconjugate, including the conjugation with antibody, peptide, aptamer, small chemical, lipidoid, cell-penetrating peptide polymer, and nanoparticle. These siRNA bioconjugate, either administrated alone or formulated with other agents, could significantly improve pharmacokinetic behavior, enhance the biological half-life, and increase the targetability while maintaining sufficient gene silencing activity, with a concomitant improvement of the therapeutic outcomes and diminishment of adverse effects. This review emphasizes the delivery application of these siRNA bioconjugates, especially the conjugation strategy that control the integrity, stability and release of siRNA bioconjugates. The limitations conferred by these conjugation strategies have also been covered.
Keywords: asymmetric siRNA; lipid siRNA conjugate; siRNA bioconjugate; siRNA scaffold; spherical siRNA.
Conflict of interest statement
The author declares no conflict of interest.
Figures





References
-
- Jenny K.W., Lam A.J.W. What is the Future of SiRNA Therapeutics. J. Drug Des. Res. 2014;1:1005.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

