Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI
- PMID: 31200518
- PMCID: PMC6631171
- DOI: 10.3390/nano9060879
Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI
Abstract
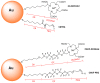
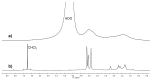
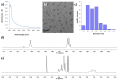
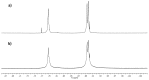
Gold nanoparticles carrying fluorinated ligands in their monolayer are, by themselves, contrast agents for 19F magnetic resonance imaging displaying high sensitivity because of the high density of fluorine nuclei achievable by grafting suitable ligands on the gold core surface. Functionalization of these nanoparticles with Gd(III) chelates allows adding a further functional activity to these systems, developing materials also acting as contrast agents for proton magnetic resonance imaging. These dual mode contrast agents may allow capitalizing on the benefits of 1H and 19F magnetic resonance imaging in a single diagnostic session. In this work, we describe a proof of principle of this approach by studying these nanoparticles in a high field preclinical scanner. The Gd(III) centers within the nanoparticles monolayer shorten considerably the 19F T1 of the ligands but, nevertheless, these systems display strong and sharp NMR signals which allow recording good quality 19F MRI phantom images at nanoparticle concentration of 20 mg/mL after proper adjustment of the imaging sequence. The Gd(III) centers also influence the T1 relaxation time of the water protons and high quality 1H MRI images could be obtained. Gold nanoparticles protected by hydrogenated ligands and decorated with Gd(III) chelates are reported for comparison as 1H MRI contrast agents.
Keywords: 19F MRI; contrast agents; fluorinated monolayers; fluorine; gadolinium; hybrid organic-inorganic nanoparticles; magnetic resonance imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Relaxometric studies of gadolinium-functionalized perfluorocarbon nanoparticles for MR imaging.Contrast Media Mol Imaging. 2014 Jan-Feb;9(1):83-91. doi: 10.1002/cmmi.1541. Contrast Media Mol Imaging. 2014. PMID: 24470297
-
Fluorinated Mesoporous Silica Nanoparticles for Binuclear Probes in 1H and 19F Magnetic Resonance Imaging.Langmuir. 2017 Oct 10;33(40):10531-10542. doi: 10.1021/acs.langmuir.7b01792. Epub 2017 Sep 26. Langmuir. 2017. PMID: 28869376
-
Redox ferrocenylseleno compounds modulate longitudinal and transverse relaxation times of FNPs-Gd MRI contrast agents for multimodal imaging and photo-Fenton therapy.Acta Biomater. 2023 Jul 1;164:496-510. doi: 10.1016/j.actbio.2023.04.006. Epub 2023 Apr 11. Acta Biomater. 2023. PMID: 37054962
-
Cy5.5-Labeled and gadolinium-chelated chitosan nanoparticles.2010 Sep 2 [updated 2010 Oct 20]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2010 Sep 2 [updated 2010 Oct 20]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21028752 Free Books & Documents. Review.
-
Pushing the sensitivity envelope of lanthanide-based magnetic resonance imaging (MRI) contrast agents for molecular imaging applications.Acc Chem Res. 2009 Jul 21;42(7):822-31. doi: 10.1021/ar800192p. Acc Chem Res. 2009. PMID: 19534516 Review.
Cited by
-
Synthesis and Stabilization of Support-Free Mesoporous Gold Nanoparticles.Nanomaterials (Basel). 2020 Jun 3;10(6):1107. doi: 10.3390/nano10061107. Nanomaterials (Basel). 2020. PMID: 32503247 Free PMC article.
-
Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics.Materials (Basel). 2020 Jan 22;13(3):513. doi: 10.3390/ma13030513. Materials (Basel). 2020. PMID: 31978954 Free PMC article.
-
Current Overview of Metal Nanoparticles' Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles.Pharmaceuticals (Basel). 2023 Oct 4;16(10):1410. doi: 10.3390/ph16101410. Pharmaceuticals (Basel). 2023. PMID: 37895881 Free PMC article. Review.
-
Utilization of nanomaterials in MRI contrast agents and their role in therapy guided by imaging.Front Bioeng Biotechnol. 2024 Nov 19;12:1484577. doi: 10.3389/fbioe.2024.1484577. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39628648 Free PMC article. Review.
-
19 F MRI Nanotheranostics for Cancer Management: Progress and Prospects.ChemMedChem. 2022 Feb 16;17(4):e202100701. doi: 10.1002/cmdc.202100701. Epub 2022 Jan 12. ChemMedChem. 2022. PMID: 34951121 Free PMC article. Review.
References
-
- Marson D., Guida F., Şologan M., Boccardo S., Pengo P., Perissinotto F., Iacuzzi V., Pellizzoni E., Polizzi S., Casalis L., et al. Mixed Fluorinated/Hydrogenated Self-Assembled Monolayer-Protected Gold Nanoparticles: In Silico and In Vitro Behavior. Small. 2019;15:1900323. doi: 10.1002/smll.201900323. - DOI - PubMed
-
- Wu Y., Ali M.R.K., Chen K., Fang N., El-Sayed M.A. Gold nanoparticles in biological optical imaging. Nano Today. 2019;24:120–140. doi: 10.1016/j.nantod.2018.12.006. - DOI
LinkOut - more resources
Full Text Sources

