Hyperosmotic Stress Response Memory is Modulated by Gene Positioning in Yeast
- PMID: 31200564
- PMCID: PMC6627694
- DOI: 10.3390/cells8060582
Hyperosmotic Stress Response Memory is Modulated by Gene Positioning in Yeast
Abstract
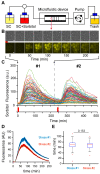
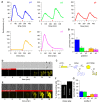
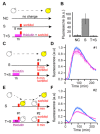
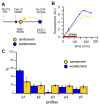
Cellular memory is a critical ability that allows microorganisms to adapt to potentially detrimental environmental fluctuations. In the unicellular eukaryote Saccharomyces cerevisiae, cellular memory can take the form of faster or slower responses within the cell population to repeated stresses. Using microfluidics and fluorescence time-lapse microscopy, we studied how yeast responds to short, pulsed hyperosmotic stresses at the single-cell level by analyzing the dynamic behavior of the stress-responsive STL1 promoter (pSTL1) fused to a fluorescent reporter. We established that pSTL1 exhibits variable successive activation patterns following two repeated short stresses. Despite this variability, most cells exhibited a memory of the first stress as decreased pSTL1 activity in response to the second stress. Notably, we showed that genomic location is important for the memory effect, since displacement of the promoter to a pericentromeric chromatin domain decreased the transcriptional strength of pSTL1 and led to a loss of memory. This study provides a quantitative description of a cellular memory that includes single-cell variability and highlights the contribution of chromatin structure to stress memory.
Keywords: cellular memory; chromosome organization; single cell; stress response; yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

