Multispectral Depth-Resolved Fluorescence Lifetime Spectroscopy Using SPAD Array Detectors and Fiber Probes
- PMID: 31200569
- PMCID: PMC6631026
- DOI: 10.3390/s19122678
Multispectral Depth-Resolved Fluorescence Lifetime Spectroscopy Using SPAD Array Detectors and Fiber Probes
Abstract
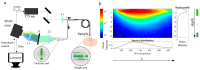
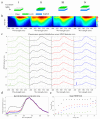
Single Photon Avalanche Diode (SPAD) arrays are increasingly exploited and have demonstrated potential in biochemical and biomedical research, both for imaging and single-point spectroscopy applications. In this study, we explore the application of SPADs together with fiber-optic-based delivery and collection geometry to realize fast and simultaneous single-point time-, spectral-, and depth-resolved fluorescence measurements at 375 nm excitation light. Spectral information is encoded across the columns of the array through grating-based dispersion, while depth information is encoded across the rows thanks to a linear arrangement of probe collecting fibers. The initial characterization and validation were realized against layered fluorescent agarose-based phantoms. To verify the practicality and feasibility of this approach in biological specimens, we measured the fluorescence signature of formalin-fixed rabbit aorta samples derived from an animal model of atherosclerosis. The initial results demonstrate that this detection configuration can report fluorescence spectral and lifetime contrast originating at different depths within the specimens. We believe that our optical scheme, based on SPAD array detectors and fiber-optic probes, constitute a powerful and versatile approach for the deployment of multidimensional fluorescence spectroscopy in clinical applications where information from deeper tissue layers is important for diagnosis.
Keywords: CMOS; SPAD; depth-resolved fluorescence; fiber optics; fluorescence lifetime; fluorescence spectroscopy; tissue diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gillenwater A., Jacob R., Ganeshappa R., Kemp B., El-Naggar A.K., Palmer J.L., Clayman G., Mitchell M.F., Richards-Kortum R. Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Arch. Otolaryngol. Head Neck Surg. 1998;124:1251–1258. doi: 10.1001/archotol.124.11.1251. - DOI - PubMed
-
- Yuvaraj M., Udayakumar K., Jayanth V., Prakasa Rao A., Bharanidharan G., Koteeswaran D., Munusamy B.D., Murali Krishna C., Ganesan S. Fluorescence spectroscopic characterization of salivary metabolites of oral cancer patients. J. Photochem. Photobiol. B Biol. 2014;130:153–160. doi: 10.1016/j.jphotobiol.2013.11.006. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

