The effect of hypoxia on chondrogenesis of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells
- PMID: 31200719
- PMCID: PMC6567476
- DOI: 10.1186/s12917-019-1954-1
The effect of hypoxia on chondrogenesis of equine synovial membrane-derived and bone marrow-derived mesenchymal stem cells
Abstract
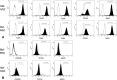
Background: Joint injury is extremely common in equine athletes and post-traumatic osteoarthritis (PTOA), a progressive and debilitating disease, is estimated to affect 60% of horses in the USA. The limited potential for intrinsic healing of articular cartilage has prompted intense efforts to identify a cell-based repair strategy to prevent progression of PTOA. Mesenchymal stem cells (MSCs) have the potential to become an ideal source for cell-based treatment of cartilage lesions; however, full chondrogenic differentiation remains elusive. Due to the relatively low oxygen tension in articular cartilage, hypoxia has been proposed as a method of increasing MSC chondrogenesis. The objective of this study was to investigate the effect of hypoxic culture conditions on chondrogenesis in equine synovial membrane-derived MSCs (SM-MSCs) and bone marrow-derived MSCs (BM-MSCs). MSCs were isolated from synovial membrane and bone marrow collected from 5 horses. Flow cytometric analysis was used to assess cell surface marker expression including CD29, CD44, CD90, CD105, CD45, CD-79α, MHCI and MHCII. MSC pellets were cultured in normoxic (21% O2) or in hypoxic (5% O2) culture conditions for 28 days. Following the culture period, chondrogenesis was assessed by histology, biochemical analyses and gene expression of chondrogenic-related genes including ACAN, COL2b, SOX9, and COL10A1.
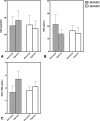
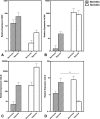
Results: Both cell types expressed markers consistent with stemness including CD29, CD44, CD90, CD105, and MHCI and were negative for exclusion markers (CD45, CD79α, and MHCII). Although the majority of outcome variables of chondrogenic differentiation were not significantly different between cell types or culture conditions, COL10A1 expression, a marker of chondrocyte hypertrophy, was lowest in hypoxic SM-MSCs and was significantly lower in hypoxic SM-MSCs compared to hypoxic BM-MSCs.
Conclusions: Hypoxic culture conditions do not appear to increase chondrogenesis of equine SM-MSCs or BM-MSCs; however, hypoxia may downregulate the hypertrophic marker COL10A1 in SM-MSCs.
Keywords: Bone marrow; Chondrogenesis; Equine; Hypoxia; Mesenchymal stem cell; Normoxia; Synovial membrane.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Respective stemness and chondrogenic potential of mesenchymal stem cells isolated from human bone marrow, synovial membrane, and synovial fluid.Stem Cell Res Ther. 2020 Jul 25;11(1):316. doi: 10.1186/s13287-020-01786-5. Stem Cell Res Ther. 2020. PMID: 32711576 Free PMC article.
-
Comparison of the Chondrogenic Differentiation Potential of Equine Synovial Membrane-Derived and Bone Marrow-Derived Mesenchymal Stem Cells.Front Vet Sci. 2019 Jun 6;6:178. doi: 10.3389/fvets.2019.00178. eCollection 2019. Front Vet Sci. 2019. PMID: 31245393 Free PMC article.
-
Autologous Platelet Lysate Does Not Enhance Chondrogenic Differentiation of Equine Bone Marrow-Derived Mesenchymal Stromal Cells Despite Increased TGF-β1 Concentration.Stem Cells Dev. 2020 Feb 1;29(3):144-155. doi: 10.1089/scd.2019.0239. Epub 2020 Jan 6. Stem Cells Dev. 2020. PMID: 31802705
-
The Importance of Physioxia in Mesenchymal Stem Cell Chondrogenesis and the Mechanisms Controlling Its Response.Int J Mol Sci. 2019 Jan 23;20(3):484. doi: 10.3390/ijms20030484. Int J Mol Sci. 2019. PMID: 30678074 Free PMC article. Review.
-
Chondrogenesis of mesenchymal stem cells: role of tissue source and inducing factors.Stem Cell Res Ther. 2010 Oct 13;1(4):31. doi: 10.1186/scrt31. Stem Cell Res Ther. 2010. PMID: 20959030 Free PMC article. Review.
Cited by
-
Comparison of the chondrogenic potential of eBMSCs and eUCMSCs in response to selected peptides and compounds.BMC Vet Res. 2025 Feb 17;21(1):70. doi: 10.1186/s12917-024-04448-3. BMC Vet Res. 2025. PMID: 39956895 Free PMC article.
-
Mesenchymal stem cells for osteoarthritis: Recent advances in related cell therapy.Bioeng Transl Med. 2024 Aug 5;10(1):e10701. doi: 10.1002/btm2.10701. eCollection 2025 Jan. Bioeng Transl Med. 2024. PMID: 39801757 Free PMC article. Review.
-
Migratory chondroprogenitors retain superior intrinsic chondrogenic potential for regenerative cartilage repair as compared to human fibronectin derived chondroprogenitors.Sci Rep. 2021 Dec 8;11(1):23685. doi: 10.1038/s41598-021-03082-5. Sci Rep. 2021. PMID: 34880351 Free PMC article.
-
DMOG Negatively Impacts Tissue Engineered Cartilage Development.Cartilage. 2021 Dec;13(2_suppl):722S-733S. doi: 10.1177/1947603520967060. Epub 2020 Oct 26. Cartilage. 2021. PMID: 33100027 Free PMC article.
-
Respective stemness and chondrogenic potential of mesenchymal stem cells isolated from human bone marrow, synovial membrane, and synovial fluid.Stem Cell Res Ther. 2020 Jul 25;11(1):316. doi: 10.1186/s13287-020-01786-5. Stem Cell Res Ther. 2020. PMID: 32711576 Free PMC article.
References
-
- Maiden L, Thjodleifsson B, Seigal A, et al. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol. 2007;5(9):1040–1045. doi: 10.1016/j.cgh.2007.04.031. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

