Improving sensitivity of amyloid detection by Congo red stain by using polarizing microscope and avoiding pitfalls
- PMID: 31200733
- PMCID: PMC6567537
- DOI: 10.1186/s13000-019-0822-4
Improving sensitivity of amyloid detection by Congo red stain by using polarizing microscope and avoiding pitfalls
Abstract
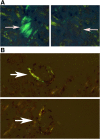
Systemic amyloidosis is a devastating group of disorders for which there is no current cure. The treatment goal is to reduce the burden of amyloidogenic protein precursors. The treatment is only effective if applied early in the disease process before significant and irreversible end organ damage has taken place. Congo red is still the standard stain used in most histopathology laboratories to identify amyloid material in tissues. The identification of Congophilic amyloid material is challenging because of multiple interfering factors. Here we describe improved sensitivity of identifying Congophilic materials in histologic sections using a metallurgical polarized microscope specifically constructed for polarized microscopy. The microscope is equipped with strain-free optics, matching polarizers, dis-integrated compensators, and a circular mobile stage. Compared to a standard clinical microscope, this setup significantly improves sensitivity of identifying amyloid material in Congo red-stained slides. We also describe the deleterious effect of plastic coverslip which can interfere with the ability to examine the slides under polarized light. We present a series of 10 different patients who had cardiac, brain, and salivary gland biopsies that were either equivocal or deemed negative using a standard clinical microscope but were positive using the equipment described above. These samples were confirmed to be positive by other methods including electron microscopy. We conclude that use of the correct equipment is needed before ruling out amyloidosis in tissue sections.
Keywords: Amyloidosis; Congo red; Metallurgical microscope; Polarized microscopy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Sipe JD, Benson MD, Buxbaum JN, Si I, Merlini G, Saraiva MJM, et al. Amyloid fibril proteins and amyloidosis: chemical identification and clinical classification International Society of Amyloidosis 2016 nomenclature guidelines. Amyloid. 2016;23(4):209–213. doi: 10.1080/13506129.2016.1257986. - DOI - PubMed
-
- Kyle RA, Greipp PR, Fallon WM. Primary systemic amyloidosis: multivariate analysis for prognostic factors in 168 cases. Blood. 1986;68(1):220. - PubMed
-
- Maixnerova D, Jancova E, Skibova J, Rysava R, Rychlik I, Viklicky O, et al. Nationwide biopsy survey of renal diseases in the Czech Republic during the years 1994-2011. J Nephrol. 2014. - PubMed

