Colorectal cancer-derived extracellular vesicles induce transformation of fibroblasts into colon carcinoma cells
- PMID: 31200749
- PMCID: PMC6567673
- DOI: 10.1186/s13046-019-1248-2
Colorectal cancer-derived extracellular vesicles induce transformation of fibroblasts into colon carcinoma cells
Abstract
Background: We reported that horizontal transfer of malignant traits to target cells is a potential pathway to explain cancer dissemination. Although these results were encouraging, they were never corroborated by data showing the molecular mechanisms responsible for the observed phenomenon.
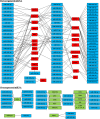
Methods: In the present study, we exposed BRCA1-KO fibroblasts to extracellular vesicles (EVs) isolated from a colon cancer cell line (HT29) and from sera of patients with colorectal cancer. Three weeks after exposure, fibroblasts were injected subcutaneously into NOD-SCID mice. Whole genome sequencing, transcriptome analysis and RNA sequencing of cancer EVs and fibroblasts prior and after exposure to cancer EVs were performed.
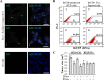
Results: Phenotypical transformation of the fibroblasts into colon cancer cells was confirmed by histopathological study of the xenotransplants. We observed that EV-mediated transfer of cancer microRNAs was responsible for the transition from a mesenchymal to an epithelial phenotype (MET) in the treated fibroblasts as well as activation of cell cycle progression and cell survival pathways. DNA and RNA sequencing suggested that cancer DNA was transferred and possibly transcribed in target cells. Furthermore, injection of colon cancer EVs in the tail vein of NOD-SCID mice determined neoplastic transformation and metastases in the lungs of the mice confirming for the first time the hypothesis that transfer of malignant epithelial cancer traits to distant target cells is a concept applicable to in vivo models.
Conclusions: These discoveries shed new light into the molecular mechanisms behind the horizontal transfer of malignant traits and confirm the notion that metastatic disease might be reproduced through transfer of circulating genetic material.
Keywords: Colorectal Cancer; Extracellular vesicles; Genetic material; Horizontal transfer; Metastasis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

