Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the Global Rotavirus Surveillance Network
- PMID: 31200889
- PMCID: PMC7336990
- DOI: 10.1016/S2214-109X(19)30207-4
Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008-16: findings from the Global Rotavirus Surveillance Network
Abstract
Background: Rotavirus vaccine use in national immunisation programmes has led to declines in hospital admissions for rotavirus gastroenteritis among children; however, the global impact of rotavirus vaccine introduction has not been described using primary data. We describe the impact of rotavirus vaccine introduction on admissions for acute rotavirus gastroenteritis in primarily low-income and middle-income countries, using 9 years of data from the WHO-coordinated Global Rotavirus Surveillance Network (GRSN).
Methods: Between Jan 1, 2008, and Dec 31, 2016, children younger than 5 years of age who were admitted to hospital with acute gastroenteritis were prospectively enrolled in GRSN sites. We included sites that enrolled children and collected stool specimens monthly and tested at least 100 specimens annually in the impact analysis, with a separate analysis taking into account site continuity. We compared proportions of acute gastroenteritis cases positive for rotavirus in the pre-vaccine and post-vaccine periods and calculated mean proportion changes for WHO regions, with 95% CIs; these findings were then compared with interrupted time series analyses. We did further sensitivity analyses to account for rotavirus vaccination coverage levels and sites that collected specimens for at least 11 months per year and tested at least 80 specimens per year. We also analysed the age distribution of rotavirus-positive cases before and after vaccine introduction.
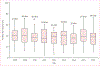
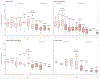
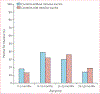
Findings: 403 140 children younger than 5 years of age admitted to hospital with acute gastroenteritis from 349 sites in 82 countries were enrolled over the study period, of whom 132 736 (32·9%) were positive for rotavirus. We included 305 789 children from 198 sites in 69 countries in the impact analysis. In countries that had not introduced rotavirus vaccine in their national immunisation programmes, rotavirus was detected in 38·0% (95% CI 4·8-73·4) of admissions for acute gastroenteritis annually whereas in those that have introduced the vaccine, rotavirus was detected in 23·0% (0·7-57·7) of admissions for acute gastroenteritis, showing a 39·6% (35·4-43·8) relative decline following introduction. Interrupted time series analyses confirmed these findings. Reductions by WHO regions ranged from 26·4% (15·0-37·8) in the Eastern Mediterranean Region to 55·2% (43·0-67·4) in the European Region and were sustained in nine countries (contributing up to 31 sites) for 6-10 years. The age distribution of children with rotavirus gastroenteritis shifted towards older children after rotavirus vaccine introduction.
Interpretation: A significant and sustained reduction in the proportion of hospital admissions for acute gastroenteritis due to rotavirus was seen among children younger than 5 years in GRSN sites following rotavirus vaccine introduction. These findings highlight the need to incorporate rotavirus vaccines into immunisation programmes in countries that have not yet introduced them and underline the importance of high-quality surveillance.
Funding: The GRSN receives funding from Gavi, the Vaccine Alliance and the US Centers for Disease Control and Prevention. No specific funding was provided for this Article.
© 2019 World Health Organization; licensee Elsevier. This is an Open Access article published under the CC BY-NC-ND 3.0 IGO license which permits users to download and share the article for non-commercial purposes, so long as the article is reproduced in the whole without changes, and provided the original source is properly cited. This article shall not be used or reproduced in association with the promotion of commercial products, services, or any entity. There should be no suggestion that WHO endorses any specific organisation, products, or services. The use of the WHO logo is not permitted. This notice should be preserved along with the article's original URL.
Figures


Comment in
-
Monitoring the impact of rotavirus vaccines on a global scale.Lancet Glob Health. 2019 Jul;7(7):e817-e818. doi: 10.1016/S2214-109X(19)30232-3. Lancet Glob Health. 2019. PMID: 31200877 No abstract available.
References
-
- Richardson V, Parashar U, Patel M. Childhood diarrhea deaths after rotavirus vaccination in Mexico. N Engl J Med 2011; 365: 772–73. - PubMed
-
- Lanzieri TM, Linhares AC, Costa I, et al. Impact of rotavirus vaccination on childhood deaths from diarrhea in Brazil. Int J Infect Dis 2011; 15: e206–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

