Mechanochemical Coupling and Junctional Forces during Collective Cell Migration
- PMID: 31200935
- PMCID: PMC6626874
- DOI: 10.1016/j.bpj.2019.05.020
Mechanochemical Coupling and Junctional Forces during Collective Cell Migration
Abstract
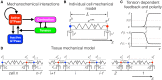
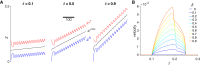
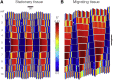
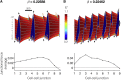
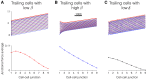
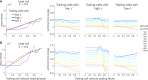
Cell migration, a fundamental physiological process in which cells sense and move through their surrounding physical environment, plays a critical role in development and tissue formation, as well as pathological processes, such as cancer metastasis and wound healing. During cell migration, dynamics are governed by the bidirectional interplay between cell-generated mechanical forces and the activity of Rho GTPases, a family of small GTP-binding proteins that regulate actin cytoskeleton assembly and cellular contractility. These interactions are inherently more complex during the collective migration of mechanically coupled cells because of the additional regulation of cell-cell junctional forces. In this study, we adapted a recent minimal modeling framework to simulate the interactions between mechanochemical signaling in individual cells and interactions with cell-cell junctional forces during collective cell migration. We find that migration of individual cells depends on the feedback between mechanical tension and Rho GTPase activity in a biphasic manner. During collective cell migration, waves of Rho GTPase activity mediate mechanical contraction/extension and thus synchronization throughout the tissue. Further, cell-cell junctional forces exhibit distinct spatial patterns during collective cell migration, with larger forces near the leading edge. Larger junctional force magnitudes are associated with faster collective cell migration and larger tissue size. Simulations of heterogeneous tissue migration exhibit a complex dependence on the properties of both leading and trailing cells. Computational predictions demonstrate that collective cell migration depends on both the emergent dynamics and interactions between cellular-level Rho GTPase activity and contractility and multicellular-level junctional forces.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
P-cadherin-mediated Rho GTPase regulation during collective cell migration.Small GTPases. 2016 Jul 2;7(3):156-63. doi: 10.1080/21541248.2016.1173772. Epub 2016 May 6. Small GTPases. 2016. PMID: 27152729 Free PMC article. Review.
-
A molecular mechanotransduction pathway regulates collective migration of epithelial cells.Nat Cell Biol. 2015 Mar;17(3):276-87. doi: 10.1038/ncb3115. Epub 2015 Feb 23. Nat Cell Biol. 2015. PMID: 25706233
-
Coupling mechanical tension and GTPase signaling to generate cell and tissue dynamics.Phys Biol. 2018 Apr 30;15(4):046004. doi: 10.1088/1478-3975/aab1c0. Phys Biol. 2018. PMID: 29473547
-
Rho GTPases and actomyosin: Partners in regulating epithelial cell-cell junction structure and function.Exp Cell Res. 2017 Sep 1;358(1):20-30. doi: 10.1016/j.yexcr.2017.03.053. Epub 2017 Mar 29. Exp Cell Res. 2017. PMID: 28363828 Free PMC article. Review.
-
Actomyosin contractility and collective migration: may the force be with you.Curr Opin Cell Biol. 2017 Oct;48:87-96. doi: 10.1016/j.ceb.2017.06.006. Epub 2017 Jul 15. Curr Opin Cell Biol. 2017. PMID: 28715714 Free PMC article. Review.
Cited by
-
A free boundary mechanobiological model of epithelial tissues.Proc Math Phys Eng Sci. 2020 Nov;476(2243):20200528. doi: 10.1098/rspa.2020.0528. Epub 2020 Nov 18. Proc Math Phys Eng Sci. 2020. PMID: 33362419 Free PMC article.
-
MARK2 regulates directed cell migration through modulation of myosin II contractility and focal adhesion organization.Curr Biol. 2022 Jun 20;32(12):2704-2718.e6. doi: 10.1016/j.cub.2022.04.088. Epub 2022 May 19. Curr Biol. 2022. PMID: 35594862 Free PMC article.
-
Effect of Geometric Curvature on Collective Cell Migration in Tortuous Microchannel Devices.Micromachines (Basel). 2020 Jul 2;11(7):659. doi: 10.3390/mi11070659. Micromachines (Basel). 2020. PMID: 32630662 Free PMC article.
-
Polarization wave at the onset of collective cell migration.Phys Rev E. 2019 Sep;100(3-1):032403. doi: 10.1103/PhysRevE.100.032403. Phys Rev E. 2019. PMID: 31640045 Free PMC article.
-
Tracking of Endothelial Cell Migration and Stiffness Measurements Reveal the Role of Cytoskeletal Dynamics.Int J Mol Sci. 2022 Jan 5;23(1):568. doi: 10.3390/ijms23010568. Int J Mol Sci. 2022. PMID: 35008993 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

