Retrospective Review of the Use of High-Dose Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone for the Treatment of Multiple Myeloma and Plasma Cell Leukemia
- PMID: 31201134
- PMCID: PMC6713607
- DOI: 10.1016/j.clml.2019.05.001
Retrospective Review of the Use of High-Dose Cyclophosphamide, Bortezomib, Doxorubicin, and Dexamethasone for the Treatment of Multiple Myeloma and Plasma Cell Leukemia
Abstract
Background: Multiple myeloma (MM) usually follows a clinical course leading to refractoriness and limited treatment options in advanced stages, which might need bridge therapies to either autologous stem cell transplantation or novel therapies. We report our experience with the high-dose chemotherapy mCBAD (modified cyclophosphamide, bortezomib, doxorubicin, and dexamethasone) regimen in newly diagnosed MM (NDMM), relapsed/refractory MM (RRMM), and plasma cell leukemia (PCL) patients.
Patients and methods: We searched our electronic records database for MM patients who received mCBAD from 2010 to 2016 for 28-day cycles of cyclophosphamide 350 mg/m2 intravenously (I.V.) twice daily with mesna 400 mg/m2 I.V. daily (days 1-4), bortezomib 1.3 mg/m2 subcutaneously/I.V. (days 1, 4, 8, 11), doxorubicin 9 mg/m2 daily continuous infusion (days 1-4), dexamethasone 40 mg orally daily (on days 1-4, 9-12, 17-20). International Myeloma Working Group (IMWG) criteria were used for response assessment and diagnosis. Descriptive statistics, Fisher exact test, χ2, Wilcoxon rank sum, and Kaplan-Meier were used for statistical purposes.
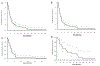
Results: One hundred forty patients met the inclusion criteria. A median of 2 cycles of therapy was administered. The overall response rate was 85% in patients with RRMM (n = 116) and 100% in NDMM (n = 13) and PCL (n = 11) patients. Respective median progression-free survival (mPFS) for NDMM, PCL, and RRMM were 19.61 months (95% confidence interval [CI], 5.26 to not applicable [NA]), 7.56 months (95% CI, 4.7 to NA), and 4.64 months (95% CI, 3.75-6.73). Patients with RRMM who used mCBAD as a bridge to autologous transplant (36.2%) had mPFS (11.48 months; 95% CI, 7.52-15.9 months) compared with those who did not (mPFS: 3.19 months; 95% CI, 2.4-3.75 months). Cytopenias occurred in more than 90% of patients, and febrile neutropenia was noted in 26%. All cases of treatment-related mortality (8%) occurred in patients with RRMM, except for 1 patient with PCL.
Conclusion: mCBAD results in high response rates in myeloma and PCL, however, with high treatment-related mortality. Its use in RRMM should be limited to patients who have immediate need for therapy without other treatment options and who have good performance status (score of 0-1) or NDMM if novel agents are not available depending on practice setting. mCBAD can be a treatment option for patients with PCL.
Keywords: High-risk MM; Multiple myeloma; Newly diagnosed MM; Relapse/refractory MM; mCBAD.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
S. Tabchi reports no conflicts of interest.
R. Nair reports no conflicts of interest.
C. Kunacheewa no conflicts of interest.
K. Patel reports no conflicts of interest
E. Manasanch has received research support from Sanofi, Quest Diagnostics, Novartis, JW Pharma, Merck; consultant fees from Takeda, Celgene, Sanofi, Amgen, BMS.
H. Lee has received consulting fees from Adaptive Biotechnologies, Celgene, Pimera and Takeda and research support from Amgen, Daiichi Sankyo, Janssen and Takeda.
D. Weber reports no conflicts of interest
S. Thomas has received consulting fees from Amgen and research support from Acerta Pharma, Amgen, Array BioPharma, Bristol-Myers-Squibb, Celgene and Idera.
B. Amini reports no conflicts of interest.
L. Feng reports no conflicts of interest.
R. Alexanian reports no conflicts of interest.
M. Qazilbash: Research support from Janssen and BioLineRx, consulting fees from Amgen.
Q. Bashir reports no conflicts of interest.
R. Mehta reports no conflicts of interest.
S. Ahmed reports no conflicts of interest
R Orlowski has received consulting fees from Amgen, Bristol-Myers-Squibb, Celgene, Janssen, Kite Pharma, Sanofi and Takeda and research support from Amgen, BioTheryX and Spectrum Pharmaceuticals.
Figures
Similar articles
-
Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial.Lancet Oncol. 2019 Jun;20(6):781-794. doi: 10.1016/S1470-2045(19)30152-4. Epub 2019 May 13. Lancet Oncol. 2019. PMID: 31097405 Clinical Trial.
-
Pomalidomide, cyclophosphamide, and dexamethasone for elderly patients with relapsed and refractory multiple myeloma: A study of the Korean Multiple Myeloma Working Party (KMMWP-164 study).Am J Hematol. 2020 Apr;95(4):413-421. doi: 10.1002/ajh.25726. Epub 2020 Jan 24. Am J Hematol. 2020. PMID: 31919872 Free PMC article. Clinical Trial.
-
Response-adapted intensification with cyclophosphamide, bortezomib, and dexamethasone versus no intensification in patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial.Lancet Haematol. 2019 Dec;6(12):e616-e629. doi: 10.1016/S2352-3026(19)30167-X. Epub 2019 Oct 14. Lancet Haematol. 2019. PMID: 31624047 Free PMC article. Clinical Trial.
-
Multiple Myeloma in the Time of COVID-19.Acta Haematol. 2020;143(5):410-416. doi: 10.1159/000507690. Epub 2020 Apr 17. Acta Haematol. 2020. PMID: 32305989 Free PMC article. Review.
-
Addition of Elotuzumab to Backbone Treatment Regimens for Multiple Myeloma: An Updated Meta-Analysis of Randomized Clinical Trials.Clin Lymphoma Myeloma Leuk. 2025 Jan;25(1):32-44. doi: 10.1016/j.clml.2024.09.008. Epub 2024 Sep 21. Clin Lymphoma Myeloma Leuk. 2025. PMID: 39414558
Cited by
-
Hyperfractionated Cyclophosphamide and Dexamethasone Alone or in Combination with Daratumumab and/or Carfilzomib for the Treatment of Relapsed or Refractory Multiple Myeloma: A Single-Center Retrospective Analysis.Clin Lymphoma Myeloma Leuk. 2023 Apr;23(4):279-290. doi: 10.1016/j.clml.2022.12.004. Epub 2022 Dec 10. Clin Lymphoma Myeloma Leuk. 2023. PMID: 36797154 Free PMC article.
-
CircRERE confers the resistance of multiple myeloma to bortezomib depending on the regulation of CD47 by exerting the sponge effect on miR-152-3p.J Bone Oncol. 2021 Jul 8;30:100381. doi: 10.1016/j.jbo.2021.100381. eCollection 2021 Oct. J Bone Oncol. 2021. PMID: 34307012 Free PMC article.
-
Biomimetic 3D Environment Based on Microgels as a Model for the Generation of Drug Resistance in Multiple Myeloma.Materials (Basel). 2021 Nov 23;14(23):7121. doi: 10.3390/ma14237121. Materials (Basel). 2021. PMID: 34885273 Free PMC article.
-
Protein-Functionalized Microgel for Multiple Myeloma Cells' 3D Culture.Biomedicines. 2022 Nov 3;10(11):2797. doi: 10.3390/biomedicines10112797. Biomedicines. 2022. PMID: 36359316 Free PMC article.
-
NMNAT1 Is a Survival Factor in Actinomycin D-Induced Osteosarcoma Cell Death.Int J Mol Sci. 2021 Aug 18;22(16):8869. doi: 10.3390/ijms22168869. Int J Mol Sci. 2021. PMID: 34445574 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

