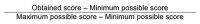Assessment of the methodological quality of local clinical practice guidelines on the identification and management of gestational diabetes
- PMID: 31201189
- PMCID: PMC6576117
- DOI: 10.1136/bmjopen-2018-027285
Assessment of the methodological quality of local clinical practice guidelines on the identification and management of gestational diabetes
Abstract
Objective: Gestational diabetes is the most common metabolic disorder of pregnancy, and it is important that well-written clinical practice guidelines (CPGs) are used to optimise healthcare delivery and improve patient outcomes. The aim of the study was to assess the methodological quality of hospital-based CPGs on the identification and management of gestational diabetes.
Design: We conducted an assessment of local clinical guidelines in English for gestational diabetes using the Appraisal of Guidelines for Research and Evaluation (AGREE II) to assess and validate methodological quality.
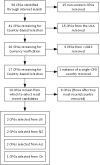
Data sources and eligibility criteria: We sought a representative selection of local CPGs accessible by the internet. Criteria for inclusion were (1) identified as a guideline, (2) written in English, (3) produced by or for the hospital in a Western country, (4) included diagnostic criteria and recommendations concerning gestational diabetes, (5) grounded on evidence-based medicine and (6) accessible over the internet. No more than two CPGs were selected from any single country.
Results: Of the 56 CPGs identified, 7 were evaluated in detail by five reviewers using the standard AGREE II instrument. Interrater variance was calculated, with strong agreement observed for those protocols considered by reviewers as the highest and lowest scoring based on the instrument. CPG results for each of the six AGREE II domains are presented categorically using a 5-point Likert scale. Only one CPG scored above average in five or more of the domains. Overall scores ranged from 91.6 (the strongest) to 50 (the weakest). Significant variation existed in the methodological quality of CPGs, even though they followed the guideline of an advising body. Specifically, appropriate identification of the evidence relied on to inform clinical decision making in CPGs was poor, as was evidence of user involvement in the development of the guideline, resource implications, documentation of competing interests of the guideline development group and evidence of external review.
Conclusions: The limitations described are important considerations for updating current and new CPGs.
Keywords: clinical practice guidelines; diabetes in pregnancy; gestational diabetes mellitus.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None Declared.
Figures
Similar articles
-
A systematic review of the quality of clinical practice guidelines for lymphedema, as assessed using the Appraisal of Guidelines for Research and Evaluation II instrument.J Vasc Surg Venous Lymphat Disord. 2020 Jul;8(4):685-692. doi: 10.1016/j.jvsv.2020.04.008. Epub 2020 Apr 23. J Vasc Surg Venous Lymphat Disord. 2020. PMID: 32335331
-
Quality appraisal of gestational diabetes mellitus guidelines with AGREE II: a systematic review.BMC Pregnancy Childbirth. 2019 Dec 5;19(1):478. doi: 10.1186/s12884-019-2597-8. BMC Pregnancy Childbirth. 2019. PMID: 31805878 Free PMC article.
-
Guidelines for Medical Nutrition Therapy in Gestational Diabetes Mellitus: Systematic Review and Critical Appraisal.J Acad Nutr Diet. 2019 Aug;119(8):1320-1339. doi: 10.1016/j.jand.2019.04.002. Epub 2019 Jun 11. J Acad Nutr Diet. 2019. PMID: 31201104
-
Quality assessment of recent evidence-based clinical practice guidelines for management of type 2 diabetes mellitus in adults using the AGREE II instrument.J Eval Clin Pract. 2018 Feb;24(1):166-172. doi: 10.1111/jep.12785. Epub 2017 Sep 25. J Eval Clin Pract. 2018. PMID: 28948654 Review.
-
Quality appraisal of clinical practice guidelines for diabetes mellitus published in China between 2007 and 2017 using the AGREE II instrument.BMJ Open. 2019 Sep 4;9(9):e022392. doi: 10.1136/bmjopen-2018-022392. BMJ Open. 2019. PMID: 31488461 Free PMC article.
Cited by
-
Clinical practice guidelines for the antenatal management of dichorionic diamniotic twin pregnancies: a systematic review.BMC Pregnancy Childbirth. 2023 May 13;23(1):347. doi: 10.1186/s12884-023-05652-z. BMC Pregnancy Childbirth. 2023. PMID: 37179347 Free PMC article.
-
Effect of stress management based self-care counseling on glycemic control in women with gestational diabetes mellitus: a randomized controlled trial study.BMC Pregnancy Childbirth. 2025 Jan 22;25(1):53. doi: 10.1186/s12884-025-07138-6. BMC Pregnancy Childbirth. 2025. PMID: 39844095 Free PMC article. Clinical Trial.
-
Quality assessment of evidence-based clinical practice guidelines for the management of pregnant women with sickle cell disease using the AGREE II instrument: a systematic review.BMC Pregnancy Childbirth. 2020 Oct 7;20(1):595. doi: 10.1186/s12884-020-03241-y. BMC Pregnancy Childbirth. 2020. PMID: 33028233 Free PMC article.
-
A protocol for a systematic review of clinical practice guidelines for the antenatal management of dichorionic diamniotic twin pregnancy.HRB Open Res. 2021 Oct 22;4:115. doi: 10.12688/hrbopenres.13418.1. eCollection 2021. HRB Open Res. 2021. PMID: 38873346 Free PMC article.
-
Mind the gap: Mapping variation between national and local clinical practice guidelines for acute paediatric asthma from the United Kingdom and the Netherlands.PLoS One. 2022 May 17;17(5):e0267445. doi: 10.1371/journal.pone.0267445. eCollection 2022. PLoS One. 2022. PMID: 35580117 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources