Does a novel diagnostic pathway including blood-based risk prediction and MRI-targeted biopsies outperform prostate cancer screening using prostate-specific antigen and systematic prostate biopsies? - protocol of the randomised study STHLM3MRI
- PMID: 31201191
- PMCID: PMC6576112
- DOI: 10.1136/bmjopen-2018-027816
Does a novel diagnostic pathway including blood-based risk prediction and MRI-targeted biopsies outperform prostate cancer screening using prostate-specific antigen and systematic prostate biopsies? - protocol of the randomised study STHLM3MRI
Abstract
Introduction: Prostate cancer is a leading cause of cancer death among men in the Western world. Early detection of prostate cancer has been shown to decrease mortality, but has limitations with low specificity leading to unnecessary biopsies and overdiagnosis of low-risk cancers. The STHLM3 trial has paved way for improved specificity in early detection of prostate cancer using the blood-based STHLM3 test for identifying men at increased risk of harbouring significant prostate cancer. Targeted prostate biopsies based on MRI images have shown non-inferior sensitivity to detect significant prostate cancer and decrease the number of biopsies and non-significant cancers among men referred for prostate biopsy in clinical practice. The strategy of the STHLM3-MRI project is to study an improved diagnostic pathway including an improved blood-based test for identification of men with increased risk of prostate cancer and use of MRI to select men for diagnostic workup with targeted prostate biopsies.
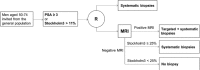
Methods: This study compares prostate cancer detection using prostate-specific antigen (PSA) and systematic biopsies to the improved pathway for prostate cancer detection using the STHLM3 test and targeted biopsies in a screening context. The study will recruit 10 000 participants during 1 June 2018 to 1 June 2020 combining a paired and randomised design. Participants are grouped by PSA and Stockholm3 test level. Men with Stockholm3 ≥11% or PSA ≥3 ng/mL are randomised to systematic or MRI-targeted biopsies. This protocol follows SPIRIT guidelines. Endpoints include the number of detected prostate cancers, number of performed biopsy procedures and number of performed MRIs. Additional aims include to assess the health economic consequences and development of automated image-analysis.
Ethics and dissemination: The study is approved by the regional ethical review board in Stockholm (2017-1280/31). The study findings will be published in peer-review journals. Findings will also be disseminated by conference/departmental presentations and by media.
Trial registration number: NCT03377881; Pre-results.
Keywords: magnetic resonance imaging; prostate disease; urological tumours.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: HG has five prostate cancer diagnostic related patents pending, has patent applications licensed to Thermo Fisher Scientific and might receive royalties from sales related to these patents. ME is named on four of these five patent applications. Karolinska Institutet collaborates with Thermo Fisher Scientific in developing the technology for the Stockholm3 test.
Figures
References
-
- Nordström T, Aly M, Clements MS, et al. . Prostate-specific antigen (PSA) testing is prevalent and increasing in Stockholm County, Sweden, Despite no recommendations for PSA screening: results from a population-based study, 2003-2011. Eur Urol 2013;63:419–25. 10.1016/j.eururo.2012.10.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous