A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia
- PMID: 31201218
- PMCID: PMC6727265
- DOI: 10.1681/ASN.2019050450
A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia
Abstract
Background: Patients with ESRD have minimal renal potassium excretion and, despite hemodialysis, often have persistent predialysis hyperkalemia. The DIALIZE study (NCT03303521) evaluated sodium zirconium cyclosilicate (SZC) in the management of hyperkalemia in hemodialysis patients.
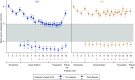
Methods: In the DIALIZE study, a double-blind, placebo-controlled, phase 3b multicenter study, we randomized adults with ESRD who were managed by three-times weekly hemodialysis and had predialysis hyperkalemia to receive placebo or SZC 5 g once daily on non-dialysis days, and titrated towards maintaining normokalemia over 4 weeks, in 5 g increments to a maximum of 15 g. The primary efficacy outcome was proportion of patients during the 4-week stable-dose evaluation period who maintained predialysis serum potassium of 4.0-5.0 mmol/L during at least three of four hemodialysis treatments after the long interdialytic interval and did not require urgent rescue therapy to reduce serum potassium.
Results: In total, 196 patients (mean [standard deviation (SD)] age =58.1 [13.7] years old) were randomized to sodium zirconium cyclosilicate or placebo. Of 97 patients receiving sodium zirconium cyclosilicate, 41.2% met the primary end point and were deemed treatment responders compared with 1.0% of 99 patients receiving placebo (P<0.001). Rescue therapy to reduce serum potassium during the treatment period was required by 2.1% of patients taking sodium zirconium cyclosilicate versus 5.1% taking placebo. Serious adverse events occurred in 7% and 8% of patients in sodium zirconium cyclosilicate and placebo groups, respectively. The two groups displayed comparable interdialytic weight gain. There were few episodes of hypokalemia.
Conclusions: Sodium zirconium cyclosilicate is an effective and well-tolerated treatment for predialysis hyperkalemia in patients with ESRD undergoing adequate hemodialysis.
Keywords: clinical trial; end-stage renal disease; hemodialysis.
Copyright © 2019 by the American Society of Nephrology.
Figures



References
-
- Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, et al. .: Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2: 999–1007, 2007 - PubMed
-
- Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB: Serum potassium levels and mortality in hemodialysis patients: A retrospective cohort study. Am J Nephrol 44: 179–186, 2016 - PubMed
-
- Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, et al. .: Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 109: 1510–1513, 2012 - PubMed
-
- USRDS: Annual data report, 2018. Available at: https://www.usrds.org/adr.aspx. Accessed March 25, 2019
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

