A Real-World, Non-interventional Indian Study Evaluating Intensive Plant-Based Butter Moisturizing Cream in Psoriasis
- PMID: 31201712
- PMCID: PMC6704203
- DOI: 10.1007/s13555-019-0307-0
A Real-World, Non-interventional Indian Study Evaluating Intensive Plant-Based Butter Moisturizing Cream in Psoriasis
Abstract
Introduction: Psoriasis is estimated to affect 0.44-2.8% of the Indian population. Moisturizers are a key adjuvant psoriasis treatment strategy, but data regarding their effectiveness, safety and compliance pattern in an Indian context are lacking. Hence, this real-world study on an intensive plant-based butter moisturizing cream (Venusia ® Max) was conducted among Indian patients with psoriasis.
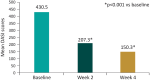
Methods: This was an observational, patient-reported outcomes (PRO) study in patients with psoriasis aged 18-75 years who were prescribed the cream in routine clinical practice, as per clinician's discretion, over 4 weeks. The primary outcome measure was improvement from baseline in quality of life assessed using the Dermatology Quality of Life Index (DLQI) at 4 weeks of the study period. The secondary outcome measures were improvement in dryness using the Dry Skin/Ichthyosis Area and Severity Index (DASI) score at 4 weeks, safety and compliance. The DLQI and DASI scores were recorded by the clinicians at baseline and after 2 (optional) and 4 weeks of starting the cream. Safety was assessed throughout the study.
Results: The study included 400 patients from 9 outpatient dermatology centers across India. Of 400 patients, 384 completed the study. A significant reduction in both the mean DLQI score (66.7%; p < 0.001) and mean DASI score (84.6%; p < 0.001) was observed at week 4 after starting the cream vs. baseline in the overall population. Overall, the cream showed a good safety and compliance profile during the study period. There were no serious adverse events or deaths.
Conclusions: The evidence from the PRO study suggests that use of the intensive plant-based butter moisturizing cream in a real-world scenario has a noticeable impact on improving the quality of life and reducing the skin dryness associated with psoriasis over 4 weeks. The moisturizing cream may serve as a valuable adjuvant treatment option for the management of psoriasis.
Trial registration number: CTRI/2017/03/008023.
Funding: Dr. Reddy's Laboratories Ltd.
Keywords: Compliance; Moisturizer; Psoriasis; Quality of life; Real world; Safety.
Conflict of interest statement
Suyog C. Mehta is a medical pharmacologist, part of the Medical Affairs team of and employee of Reddy’s Laboratories Ltd. Sujeet N. Charugulla is a medical pharmacologist, part of the Medical Affairs team and employee of Dr. Reddy’s Laboratories Ltd. Rajan Mittal is a medical pharmacologist, part of the Medical Affairs team and employee of Dr. Reddy’s Laboratories Ltd. Shivani Acharya is a medical pharmacologist, part of the Medical Affairs Team and employee of Dr. Reddy’s Laboratories Ltd. Hemangi Rajiv Jerajani received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Jayakar Thomas received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Alka Gupta received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Narasimha Rao Netha G received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Ranju Chawla received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Rashid Shaikh received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Ravindra Babu received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Irene Williams received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study. Prajakta Talathi received remuneration from Dr. Reddy’s Laboratories Ltd. for conduct of the clinical study.
Figures

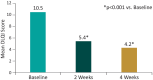
Similar articles
-
Real-World, Non-Interventional, Observational Study of Hydroxyzine Hydrochloride in Chronic Pruritus: a Prospective, Non-Comparative Study.Dermatol Ther (Heidelb). 2019 Jun;9(2):299-308. doi: 10.1007/s13555-019-0293-2. Epub 2019 Apr 4. Dermatol Ther (Heidelb). 2019. PMID: 30949959 Free PMC article.
-
Efficacy and In-Use Tolerance of Venusia Baby Moisturizer for Skin Hydration in Babies With Dry and/or Normal Skin.Cureus. 2023 Sep 11;15(9):e45032. doi: 10.7759/cureus.45032. eCollection 2023 Sep. Cureus. 2023. PMID: 37842370 Free PMC article.
-
Brodalumab and ixekizumab, anti-interleukin-17-receptor antibodies for psoriasis: a critical appraisal.Br J Dermatol. 2012 Oct;167(4):710-3; discussion 714-5. doi: 10.1111/bjd.12025. Br J Dermatol. 2012. PMID: 23013312
-
Indoor salt water baths followed by artificial ultraviolet B light for chronic plaque psoriasis.Cochrane Database Syst Rev. 2020 May 5;5(5):CD011941. doi: 10.1002/14651858.CD011941.pub2. Cochrane Database Syst Rev. 2020. PMID: 32368795 Free PMC article.
-
Antistreptococcal interventions for guttate and chronic plaque psoriasis.Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2. Cochrane Database Syst Rev. 2019. PMID: 30835819 Free PMC article.
Cited by
-
Review of natural compounds for potential psoriasis treatment.Inflammopharmacology. 2023 Jun;31(3):1183-1198. doi: 10.1007/s10787-023-01178-0. Epub 2023 Mar 30. Inflammopharmacology. 2023. PMID: 36995575 Free PMC article. Review.
References
-
- Kuchekar AB, Pujari RR, Kuchekar SB, Dhole SN, Mule PM. Psoriasis: a comprehensive review. Int J Pharm Life Sci. 2011;2:857–877.
LinkOut - more resources
Full Text Sources

