Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy
- PMID: 31201734
- PMCID: PMC6953387
- DOI: 10.1002/14651858.CD011265.pub2
Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy
Abstract
Background: Diabetic peripheral neuropathy (DPN) is a common and severe complication that affects 50% of people with diabetes. Painful DPN is reported to occur in 16% to 24% of people with diabetes. A complete and comprehensive management strategy for the prevention and treatment of DPN, whether painful or not, has not yet been defined.Research into treatment for DPN has been characterised by a series of failed clinical trials, with few noteworthy advances. Strategies that support peripheral nerve regeneration and restore neurological function in people with painful or painless DPN are needed. The amino acid acetyl-L-carnitine (ALC) plays a role in the transfer of long-chain fatty acids into mitochondria for β-oxidation. ALC supplementation also induces neuroprotective and neurotrophic effects in the peripheral nervous system. Therefore, ALC supplementation targets several mechanisms relevant to potential nerve repair and regeneration, and could have clinical therapeutic potential. There is a need for a systematic review of the evidence from clinical trials.
Objectives: To assess the effects of ALC for the treatment of DPN.
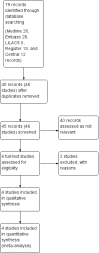
Search methods: On 2 July 2018, we searched the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, LILACS, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform. We checked references, searched citations, and contacted study authors to identify additional studies.
Selection criteria: We included randomised controlled trials (RCTs) and quasi-RCTs of ALC compared with placebo, other therapy, or no intervention in the treatment of DPN. Participants could be of any sex and age, and have type 1 or type 2 diabetes mellitus, of any severity, with painful or painless DPN. We accepted any definition of minimum criteria for DPN, in accordance with the Toronto Consensus. We imposed no language restriction.Pain was the primary outcome, measured as the proportion of participants with at least 30% (moderate) or 50% (substantial) decrease in pain over baseline, or as the score on a visual analogue scale (VAS) or Likert scale for pain.
Data collection and analysis: We followed standard Cochrane methods.
Main results: We included four studies with 907 participants, which were reported in three publications. Three trials studied ALC versus placebo (675 participants); in one trial the dose of ALC was 2000 mg/day, and in the other two trials, it was 1500 mg/day or 3000 mg/day. The fourth trial studied ALC 1500 mg/day versus methylcobalamin 1.5 mg/day (232 participants). The risk of bias was high in both trials of different ALC doses and low in the other two trials.No included trial measured the proportion of participants with at least moderate (30%) or substantial (50%) pain relief. ALC reduced pain more than placebo, measured on a 0- to 100-mm VAS (MD -9.16, 95% CI -16.76 to -1.57; three studies; 540 participants; P = 0.02; I² = 56%; random-effects; very low-certainty evidence; a higher score indicating more pain). At doses of 1500 mg/day or less, the VAS score after ALC treatment was little different from placebo (MD -0.05, 95% CI -10.00 to 9.89; two studies; 159 participants; P = 0.99; I² = 0%), but at doses greater than 1500 mg/day, ALC reduced pain more than placebo (MD -14.93, 95% CI -19.16 to -10.70; three studies; 381 participants; P < 0.00001; I² = 0%). This subgroup analysis should be viewed with caution as the evidence was even less certain than the overall analysis, which was already of very low certainty.Two placebo-controlled studies reported that vibration perception improved after 12 months. We graded this evidence as very low certainty, due to inconsistency and a high risk of bias, as the trial authors did not provide any numerical data. The placebo-controlled studies did not measure functional impairment and disability scores. No study used validated symptom scales. One study performed sensory testing, but the evidence was very uncertain.The fourth included study compared ALC with methylcobalamin, but did not report effects on pain. There was a reduction from baseline to 24 weeks in functional impairment and disability, based on the change in mean Neuropathy Disability Score (NDS; scale from zero to 10), but there was no important difference between the ALC group (mean score 1.66 ± 1.90) and the methylcobalamin group (mean score 1.35 ± 1.65) groups (P = 0.23; low-certainty evidence).One placebo-controlled study reported that six of 147 participants in the ALC > 1500 mg/day group (4.1%) and two of 147 participants in the placebo group (1.4%) discontinued treatment because of adverse events (headache, facial paraesthesia, and gastrointestinal disorders) (P = 0.17). The other two placebo-controlled studies reported no dropouts due to adverse events, and more pain, paraesthesia, and hyperaesthesias in the placebo group than the 3000 mg/day ALC group, but provided no numerical data. The overall certainty of adverse event evidence for the comparison of ALC versus placebo was low.The study comparing ALC with methylcobalamin reported that 34/117 participants (29.1%) experienced adverse events in the ALC group versus 33/115 (28.7%) in the methylcobalamin group (P = 0.95). Nine participants discontinued treatment due to adverse events (ALC: 4 participants, methylcobalamin: 5 participants), which were most commonly gastrointestinal symptoms. The certainty of the adverse event evidence for ALC versus methylcobalamin was low.Two studies were funded by the manufacturer of ALC and the other two studies had at least one co-author who was a consultant for an ALC manufacturer.
Authors' conclusions: We are very uncertain whether ALC causes a reduction in pain after 6 to 12 months' treatment in people with DPN, when compared with placebo, as the evidence is sparse and of low certainty. Data on functional and sensory impairment and symptoms are lacking, or of very low certainty. The evidence on adverse events is too uncertain to make any judgements on safety.
Conflict of interest statement
LCSPR: I gave a lecture on 23 July 2013 about the practical diagnosis of diabetic neuropathy. The talk was prepared by myself without any influence from the funders (Merck Serono).
EMKS: none known
RLGF: none known
MMA: none known
SAD: none known
Figures








Update of
- doi: 10.1002/14651858.CD011265
References
References to studies included in this review
De Grandis 2002 {published data only}
-
- Grandis D, Minardi C. Acetyl‐L‐carnitine (levacecarnine) in the treatment of diabetic neuropathy. A long‐term, randomised, double‐blind, placebo‐controlled study. Drugs in R&D 2002;3(4):223‐31. [PUBMED: 12455197] - PubMed
Li 2016 {published data only}
Sima 2005a {published data only}
-
- Sima AA, Calvani M, Mehra M, Amato A, Acetyl‐L‐Carnitine Study Group. Acetyl‐L‐carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo‐controlled trials. Diabetes Care 2005;28(1):89‐94. [PUBMED: 15616239] - PubMed
Sima 2005b {published data only}
-
- Sima AA, Calvani M, Mehra M, Amato A, Acetyl‐L‐Carnitine Study Group. Acetyl‐L‐carnitine improves pain, nerve regeneration, and vibratory perception in patients with chronic diabetic neuropathy: an analysis of two randomized placebo‐controlled trials. Diabetes Care 2005;28(1):89‐94. [PUBMED: 15616239] - PubMed
References to studies excluded from this review
Abbott 1997 {published data only}
-
- Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJM, ALCAR Foot Ulcer Study Group. Effect of treatment with acetyl‐l‐carnitine on diabetic foot ulceration in patients with peripheral neuropathy: a 1 year prospective multi‐centre study. Diabetologia 1997;40(Suppl 1):A556; Abstract no: 2184. [DOI: 10.1007/BF03162081] - DOI
Quatraro 1995 {published data only}
-
- Quatraro A, Roca P, Donzella C, Acampora R, Marfella R, Giugliano D. AcetyI‐L‐carnitine for symptomatic diabetic neuropathy. Diabetologia 1995;38:123. - PubMed
Additional references
Anon 2010
-
- Acetyl‐L‐carnitine. Monograph. Alternative Medicine Review 2010;15(1):76‐83. [PUBMED: 20359271] - PubMed
Boulton 2005a
-
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 2005;28(4):956‐62. - PubMed
Boulton 2005b
-
- Boulton AJM, Vileikyte L, Ragnarson‐Tennvall G, Apelqvis J. The global burden of diabetic foot disease. Lancet 2005;366:1719‐24. - PubMed
Boulton 2010
-
- Boulton AJM. NICE guidelines for the management of painful diabetic neuropathy. Practical Diabetes International 2010; Vol. 27, issue 6:215‐6. [DOI: 10.1002/pdi.1486] - DOI
Bril 2011
-
- Bril V, England J, Franklin GM, Backonja M, Cohen J, Toro D, et al. American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, American Academy of Physical Medicine, Rehabilitation. Evidence‐based guideline. Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011;76(20):1758‐65. [DOI: 10.1212/WNL.0b013e3182166ebe] - DOI - PMC - PubMed
Callaghan 2012
Chen 2014
-
- Chen Y, Abbate M, Tang L, Cai G, Gong Z, Wei R, et al. L‐carnitine supplementation for adults with end‐stage kidney disease requiring maintenance haemodialysis: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2014;99(2):408‐22. - PubMed
Cruccu 2004
-
- Cruccu G, Anand P, Attal N, Garcia‐Larrea L, Haanpëä M, Jørum E, et al. EFNS guidelines on neuropathic pain assessment. European Journal of Neurology 2004;11(3):153‐62. - PubMed
Daousi 2004
-
- Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabetic Medicine 2004;21(9):976‐82. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DiNicolantonio 2013
-
- DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH. L‐carnitine in the secondary prevention of cardiovascular disease: systematic review and meta‐analysis. Mayo Clinic Proceedings 2013;88(6):544‐51. - PubMed
Dyck 1988
-
- Dyck PJ. Detection, characterization, and staging of polyneuropathy: assessed in diabetics. Muscle & Nerve 1988;11(1):21‐32. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
England 2005
-
- England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. American Academy of Neurology, American Association of Electrodiagnostic Medicine, American Academy of Physical Medicine, Rehabilitation. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2005;64(2):199‐207. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 7 February 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Deeks JJ, Altman DG editor(s). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Jiang 2013
-
- Jiang Q, Jiang G, Shi KQ, Cai H, Wang YX, Zheng MH. Oral acetyl‐L‐carnitine treatment in hepatic encephalopathy: view of evidence‐based medicine. Annals of Hepatolology 2013;12(5):803‐9. - PubMed
Li 2015
PaPaS 2011
-
- Cochrane Pain, Palliative & Supportive Care Review Group. Authoring or assessing a Cochrane Protocol, Review, or Review Update for the PaPaS Review Group, 2011. Available from: papas.cochrane.org/sites/papas.cochrane.org/files/public/uploads/L%20‐%2....
Rebouche 2004
-
- Rebouche CJ. Kinetics, pharmacokinetics, and regulation of L‐carnitine and acetyl‐L‐carnitine metabolism. Annals of the New York Academy of Sciences 2004;1033:30‐41. [PUBMED: 15591001] - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rolim 2009
-
- Rolim LC, Sá JR, Chacra AR, Dib SA. Clinical heterogeneity and coexistence of diabetic neuropathies: difference and similarities between types 1 and 2 diabetes mellitus [Heterogeneidade Clínica e Coexistência da Neuropatias Diabéticas: Diferenças e Semelhanças entre Diabetes Melito Tipos 1 e 2]. Arquivos Brasileiro Endocrinologia e Metabologia 2009;53(7):818‐24. - PubMed
Rolim 2017
Scarpini 1996
-
- Scarpini E, Doneda P, Pizzul S, Chiodi P, Ramacci MT, Baron P, et al. L‐carnitine and acetyl‐L‐carnitine in human nerves from normal and diabetic subjects. Journal of the Peripheral Nervous System 1996;1(2):157‐63. - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, et al. on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Akl E, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sima 2007
-
- Sima AA. Acetyl‐L‐carnitine in diabetic polyneuropathy: experimental and clinical data. CNS Drugs 2007;21(Suppl 1):13‐23. - PubMed
Tesfaye 2010
Veronese 2017
-
- Veronese N, Sergi G, Stubbs B, Bourdel‐Marchasson I, Tessier D, Sieber C, et al. the EUGMS special interest group on diabetes. Effect of acetyl‐l‐carnitine in the treatment of diabetic peripheral neuropathy: a systematic review and meta‐analysis. European Geriatric Medicine 2017;8(2):117‐22. [DOI: 10.1016/j.eurger.2017.01.002] - DOI
Vidal‐Casariego 2013
-
- Vidal‐Casariego A, Burgos‐Peláez R, Martínez‐Faedo C, Calvo‐Gracia F, Valero‐Zanuy MÁ, Luengo‐Pérez LM, et al. Metabolic effects of L‐carnitine on type 2 diabetes mellitus: systematic review and meta‐analysis. Experimental and Clinical Endocrinology & Diabetes 2013;121(4):234‐8. - PubMed
Vileikyte 2003
-
- Vileikyte L, Peyrot M, Bundy C, Rubin RR, Leventhal H, Mora P, et al. The development and validation of a neuropathy and foot ulcer‐specific quality of life instrument. Diabetes Care 2003;26(9):2549‐55. - PubMed
Williamson 1992
-
- Williamson JR, Arrigoni‐Marteli E. The roles of glucose‐induced metabolic hypoxia and imbalances in carnitine metabolism in mediating diabetes‐induced vascular dysfunction. International Journal of Clinical Pharmacology Research 1992;12(5‐6):247‐52. - PubMed
Wong 2007
Young 1993
-
- Young MJ, Boulton AJ, MacLeod AF, Williams DRR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36(2):150‐4. - PubMed
Ziegler 2009
Zochodne 2016
-
- Zochodne DW. Sensory neurodegeneration in diabetes: beyond glucotoxicity. International Review of Neurobiology 2016;127:151‐80. [PUBMED: 27133149] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

