SIFamide peptides modulate cardiac activity differently in two species of Cancer crab
- PMID: 31201801
- PMCID: PMC6719312
- DOI: 10.1016/j.ygcen.2019.06.008
SIFamide peptides modulate cardiac activity differently in two species of Cancer crab
Abstract
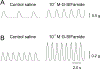
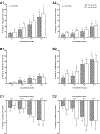
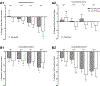
The SIFamides are a broadly conserved arthropod peptide family characterized by the C-terminal motif -SIFamide. In decapod crustaceans, two isoforms of SIFamide are known, GYRKPPFNGSIFamide (Gly1-SIFamide), which is nearly ubiquitously conserved in the order, and VYRKPPFNGSIFamide (Val1-SIFamide), known only from members of the astacidean genus Homarus. While much work has focused on the identification of SIFamide isoforms in decapods, there are few direct demonstrations of physiological function for members of the peptide family in this taxon. Here, we assessed the effects of Gly1- and Val1-SIFamide on the cardiac neuromuscular system of two closely related species of Cancer crab, Cancer borealis and Cancer irroratus. In each species, both peptides were cardioactive, with identical, dose-dependent effects elicited by both isoforms in a given species. Threshold concentrations for bioactivity are in the range typically associated with hormonal delivery, i.e., 10-9 to 10-8 M. Interestingly, and quite surprisingly, while the predicted effects of SIFamide on cardiac output are similar in both C. borealis and C. irroratus, frequency effects predominate in C. borealis, while amplitude effects predominate in C. irroratus. These findings suggest that, while SIFamide is likely to increase cardiac output in both crabs, the mechanism through which this is achieved is different in the two species. Immunohistochemical/mass spectrometric data suggest that SIFamide is delivered to the heart hormonally rather than locally, with the source of hormonal release being midgut epithelial endocrine cells in both Cancer species. If so, midgut-derived SIFamide may function as a regulator of cardiac output during the process of digestion.
Keywords: Cardiac neuromuscular system; Cardiotropic peptide; Central pattern generator; Crustacea; Midgut epithelial endocrine signaling; Peptide hormone.
Copyright © 2019. Published by Elsevier Inc.
Figures
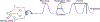


Similar articles
-
Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus.J Exp Biol. 2007 Feb;210(Pt 4):699-714. doi: 10.1242/jeb.02696. J Exp Biol. 2007. PMID: 17267655
-
SIFamide peptides in clawed lobsters and freshwater crayfish (Crustacea, Decapoda, Astacidea): a combined molecular, mass spectrometric and electrophysiological investigation.Gen Comp Endocrinol. 2008 Apr 1;156(2):347-60. doi: 10.1016/j.ygcen.2008.01.011. Epub 2008 Jan 26. Gen Comp Endocrinol. 2008. PMID: 18308319
-
Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val1)-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus.J Comp Neurol. 2006 May 20;496(3):406-21. doi: 10.1002/cne.20932. J Comp Neurol. 2006. PMID: 16566002
-
SIFamide illustrates the rapid evolution in Arthropod neuropeptide research.Gen Comp Endocrinol. 2009 May 15;162(1):27-35. doi: 10.1016/j.ygcen.2008.10.020. Epub 2008 Oct 26. Gen Comp Endocrinol. 2009. PMID: 19014945 Review.
-
Evolution of gonadotropin-inhibitory hormone receptor and its ligand.Gen Comp Endocrinol. 2014 Dec 1;209:148-61. doi: 10.1016/j.ygcen.2014.09.002. Epub 2014 Sep 16. Gen Comp Endocrinol. 2014. PMID: 25220854 Review.
Cited by
-
Neuropeptidomic Profiling and Localization in the Crustacean Cardiac Ganglion Using Mass Spectrometry Imaging with Multiple Platforms.J Am Soc Mass Spectrom. 2020 Dec 2;31(12):2469-2478. doi: 10.1021/jasms.0c00191. Epub 2020 Aug 11. J Am Soc Mass Spectrom. 2020. PMID: 33595330 Free PMC article.
-
Neuropeptidomics of the American Lobster Homarus americanus.J Proteome Res. 2024 May 3;23(5):1757-1767. doi: 10.1021/acs.jproteome.3c00925. Epub 2024 Apr 22. J Proteome Res. 2024. PMID: 38644788 Free PMC article.
-
Decoding Neuropeptide Complexity: Advancing Neurobiological Insights from Invertebrates to Vertebrates through Evolutionary Perspectives.ACS Chem Neurosci. 2025 May 7;16(9):1662-1679. doi: 10.1021/acschemneuro.5c00053. Epub 2025 Apr 22. ACS Chem Neurosci. 2025. PMID: 40261092 Free PMC article. Review.
-
Mass Spectrometry Quantification, Localization, and Discovery of Feeding-Related Neuropeptides in Cancer borealis.ACS Chem Neurosci. 2021 Feb 17;12(4):782-798. doi: 10.1021/acschemneuro.1c00007. Epub 2021 Feb 1. ACS Chem Neurosci. 2021. PMID: 33522802 Free PMC article.
References
-
- Braga VH, Armelin VA, Teixeira MT, Abe AS, Rantin FT, Florindo LH, 2016. The effects of feeding on cardiac control of the broad-nosed caiman (Caiman latirostris): the role of the autonomic nervous system and NANC factors. J Exp Zool A Ecol Genet Physiol 325, 524–531. - PubMed
-
- Brezina V, Orekhova IV, Weiss KR, 2000. The neuromuscular transform: The dynamic, nonlinear link between motor neuron firing patterns and muscle contraction in rhythmic behaviors. Journal of Neurophysiology 83, 207–231. - PubMed
-
- Brezina V, Weiss KR, 2000. The neuromuscular transform constrains the production of functional rhythmic behaviors. Journal of Neurophysiology 83, 232–259. - PubMed
-
- Christie AE, 2011. Crustacean neuroendocrine systems and their signaling agents. Cell Tissue Res 345, 41–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

