Pain and activity after vaginal reconstructive surgery for pelvic organ prolapse and stress urinary incontinence
- PMID: 31201809
- PMCID: PMC7250460
- DOI: 10.1016/j.ajog.2019.06.004
Pain and activity after vaginal reconstructive surgery for pelvic organ prolapse and stress urinary incontinence
Abstract
Background: Little is known about short- and long-term pain and functional activity after surgery for pelvic organ prolapse.
Objective: The objectives of the study were to describe postoperative pain and functional activity after transvaginal native tissue reconstructive surgery with apical suspension and retropubic synthetic midurethral sling and to compare these outcomes between patients receiving 2 common transvaginal prolapse repairs, uterosacral ligament, and sacrospinous ligament vaginal vault suspension.
Study design: This planned secondary analysis of a 2 × 2 factorial randomized trial included 374 women randomized to receive uterosacral (n = 188) or sacrospinous (n = 186) vaginal vault suspension to treat both stages 2-4 apical vaginal prolapse and stress urinary incontinence between 2008 and 2013 at 9 medical centers. Participants were also randomized to receive perioperative pelvic muscle therapy or usual care. All patients received transvaginal native tissue repairs and a midurethral sling. Participants completed the Surgical Pain Scales (0-10 numeric rating scales; higher scores = greater pain) and Activity Assessment Scale (0-100; higher score = higher activity) prior to surgery and at 2 weeks, 4-6 weeks, and 3 months postoperatively. The MOS 36-item Short-Form Health Survey was completed at baseline and 6, 12, and 24 months after surgery; the bodily pain, physical functioning, and role-physical subscales were used for this analysis (higher scores = less disability). Self-reported pain medication use was also collected.
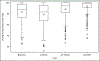
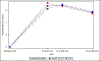
Results: Before surgery, average pain at rest and during normal activity were (adjusted mean ± SE) 2.24 ± 0.23 and 2.76 ± 0.25; both increased slightly from baseline at 2 weeks (+0.65, P = .004, and +0.74, P = .007, respectively) and then decreased below baseline at 3 months (-0.87 and -1.14, respectively, P < .001), with no differences between surgical groups. Pain during exercise/strenuous activity and worst pain decreased below baseline levels at 4-6 weeks (-1.26, P = .014, and -0.95, P = .002) and 3 months (-1.97 and -1.50, P < .001) without differences between surgical groups. Functional activity as measured by the Activity Assessment Scale improved from baseline at 4-6 weeks (+9.24, P < .001) and 3 months (+13.79, P < .001). The MOS 36-item Short-Form Health Survey Bodily Pain, Physical Functioning, and Role-Physical Scales demonstrated significant improvements from baseline at 6, 12, and 24 months (24 months: +5.62, +5.79, and +4.72, respectively, P < .001 for each) with no differences between groups. Use of narcotic pain medications was reported by 14.3% of participants prior to surgery and 53.7% at 2 and 26.1% at 4-6 weeks postoperatively; thereafter use was similar to baseline rates until 24 months when it decreased to 6.8%. Use of nonnarcotic pain medication was reported by 48.1% of participants prior to surgery, 68.7% at 2 weeks, and similar to baseline at 3 months; thereafter use dropped steadily to 26.6% at 2 years. Uterosacral ligament suspension resulted in less new or worsening buttock pain than sacrospinous suspension at 4-6 weeks postoperatively (4.6% vs 10.5%, P = .043) but no difference in groin or thigh pain.
Conclusion: Pain and functional activity improve for up to 2 years after native tissue reconstructive surgery with uterosacral or sacrospinous vaginal vault suspension and midurethral sling for stages 2-4 pelvic organ prolapse. On average, immediate postoperative pain is low and improves to below baseline levels by 4-6 weeks.
Keywords: functional activity; pelvic floor disorders; pelvic organ prolapse; postoperative pain; sacrospinous ligament fixation; stress urinary incontinence; uterosacral ligament suspension; vaginal reconstructive surgery.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures













Comment in
-
Prolapse, pain, and pelvic floor muscle dysfunction.Am J Obstet Gynecol. 2019 Sep;221(3):177-178. doi: 10.1016/j.ajog.2019.07.038. Am J Obstet Gynecol. 2019. PMID: 31492376 No abstract available.
Similar articles
-
Comparison of 2 transvaginal surgical approaches and perioperative behavioral therapy for apical vaginal prolapse: the OPTIMAL randomized trial.JAMA. 2014 Mar 12;311(10):1023-34. doi: 10.1001/jama.2014.1719. JAMA. 2014. PMID: 24618964 Free PMC article. Clinical Trial.
-
Effect of Uterosacral Ligament Suspension vs Sacrospinous Ligament Fixation With or Without Perioperative Behavioral Therapy for Pelvic Organ Vaginal Prolapse on Surgical Outcomes and Prolapse Symptoms at 5 Years in the OPTIMAL Randomized Clinical Trial.JAMA. 2018 Apr 17;319(15):1554-1565. doi: 10.1001/jama.2018.2827. JAMA. 2018. PMID: 29677302 Free PMC article. Clinical Trial.
-
Association between adjuvant posterior repair and success of native tissue apical suspension.Am J Obstet Gynecol. 2020 Feb;222(2):161.e1-161.e8. doi: 10.1016/j.ajog.2019.08.024. Epub 2019 Aug 23. Am J Obstet Gynecol. 2020. PMID: 31449806 Free PMC article.
-
Guideline No. 413: Surgical Management of Apical Pelvic Organ Prolapse in Women.J Obstet Gynaecol Can. 2021 Apr;43(4):511-523.e1. doi: 10.1016/j.jogc.2021.02.001. Epub 2021 Feb 3. J Obstet Gynaecol Can. 2021. PMID: 33548503
-
Surgery in urogynecology.Minerva Med. 2012 Feb;103(1):23-36. Minerva Med. 2012. PMID: 22278066 Review.
Cited by
-
Comparing Post-Operative Pain Outcomes in Pelvic Organ Prolapse Surgery With and Without a Hysterectomy.Int Urogynecol J. 2025 May 10. doi: 10.1007/s00192-025-06162-6. Online ahead of print. Int Urogynecol J. 2025. PMID: 40347243
-
PROMIS pain intensity and interference after pelvic organ prolapse surgery.Int Urogynecol J. 2023 Oct;34(10):2415-2420. doi: 10.1007/s00192-023-05547-9. Epub 2023 May 5. Int Urogynecol J. 2023. PMID: 37145124
-
Surgical outcomes of sacrospinous hysteropexy and hysteropreservation for pelvic organ prolapse: a systematic review of randomized controlled trials.Front Med (Lausanne). 2024 Jul 24;11:1399247. doi: 10.3389/fmed.2024.1399247. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39114831 Free PMC article.
-
The Interplay Between Chronic Pelvic Pain and Pelvic Organ Prolapse.Int Urogynecol J. 2025 Mar;36(3):523-531. doi: 10.1007/s00192-024-06040-7. Epub 2025 Feb 4. Int Urogynecol J. 2025. PMID: 39903235
-
International urogynecological consultation chapter 4.1: definition of outcomes for pelvic organ prolapse surgery.Int Urogynecol J. 2023 Nov;34(11):2689-2699. doi: 10.1007/s00192-023-05660-9. Epub 2023 Oct 11. Int Urogynecol J. 2023. PMID: 37819369 Review.
References
-
- FDA. Urogynecologic surgical mesh:update on the safety and effectiveness of transvaginal mesh placement for pelvic organ prolapse, 2011.
-
- Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol 2003;188:108–15. - PubMed
-
- Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E. Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol 2002;186:712–6. - PubMed
-
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HD041261/HD/NICHD NIH HHS/United States
- U01 HD041249/HD/NICHD NIH HHS/United States
- U10 HD054215/HD/NICHD NIH HHS/United States
- U10 HD054214/HD/NICHD NIH HHS/United States
- U01 HD069031/HD/NICHD NIH HHS/United States
- U10 HD041267/HD/NICHD NIH HHS/United States
- UG1 HD054214/HD/NICHD NIH HHS/United States
- U10 HD054241/HD/NICHD NIH HHS/United States
- U10 HD041250/HD/NICHD NIH HHS/United States
- UG1 HD041267/HD/NICHD NIH HHS/United States
- U10 HD054136/HD/NICHD NIH HHS/United States
- UG1 HD041261/HD/NICHD NIH HHS/United States
- U24 HD069031/HD/NICHD NIH HHS/United States
- UG1 HD054241/HD/NICHD NIH HHS/United States

