Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury
- PMID: 31201834
- PMCID: PMC6773499
- DOI: 10.1016/j.jhep.2019.05.029
Activation of YAP attenuates hepatic damage and fibrosis in liver ischemia-reperfusion injury
Abstract
Background & aims: Hepatic ischemia-reperfusion injury (IRI) is a major complication of hemorrhagic shock, liver resection and transplantation. YAP, a key downstream effector of the Hippo pathway, is essential for determining cell fate and maintaining homeostasis in the liver. We aimed to elucidate its role in IRI.
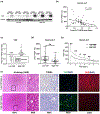
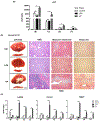
Methods: The role of YAP/Hippo signaling was systematically studied in biopsy specimens from 60 patients after orthotopic liver transplantation (OLT), and in a mouse model of liver warm IRI. Human biopsy specimens were collected after 2-10 h of cold storage and 3 h post-reperfusion, before being screened by western blot. In the mouse model, the role of YAP was probed by activating or inhibiting YAP prior to ischemia-reperfusion.
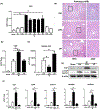
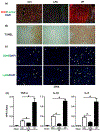
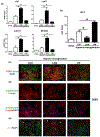
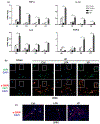
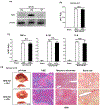
Results: In human biopsies, high post-OLT YAP expression was correlated with well-preserved histology and improved hepatocellular function at postoperative day 1-7. In mice, the ischemia insult (90 min) triggered intrinsic hepatic YAP expression, which peaked at 1-6 h of reperfusion. Activation of YAP protected the liver against IR-stress, by promoting regenerative and anti-oxidative gene induction, while diminishing oxidative stress, necrosis/apoptosis and the innate inflammatory response. Inhibition of YAP aggravated hepatic IRI and suppressed repair/anti-oxidative genes. In mouse hepatocyte cultures, activating YAP prevented hypoxia-reoxygenation induced stress. Interestingly, YAP activation suppressed extracellular matrix synthesis and diminished hepatic stellate cell (HSC) activation, whereas YAP inhibition significantly delayed hepatic repair, potentiated HSC activation, and enhanced liver fibrosis at 7 days post-IRI. Notably, YAP activation failed to protect Nrf2-deficient livers against IR-mediated damage, leading to extensive fibrosis.
Conclusion: Our novel findings document the crucial role of YAP in IR-mediated hepatocellular damage and liver fibrogenesis, providing evidence of a potential therapeutic target for the management of sterile liver inflammation in transplant recipients.
Lay summary: In the clinical arm, graft YAP expression negatively correlated with liver function and tissue damage after human liver transplantation. YAP activation attenuated hepatocellular oxidative stress and diminished the innate immune response in mouse livers following ischemia-reperfusion injury. In the mouse model, YAP inhibited hepatic stellate cell activation, and abolished injury-mediated fibrogenesis up to 7 days after the ischemic insult.
Keywords: Fibrogenesis; Hippo; Immune response; Inflammation; Liver ischemia-reperfusion injury; Orthotopic liver transplantation; YAP.
Copyright © 2019 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
Myofibroblast YAP/TAZ is dispensable for liver fibrosis in mice.J Hepatol. 2021 Jul;75(1):238-241. doi: 10.1016/j.jhep.2021.02.026. Epub 2021 Mar 3. J Hepatol. 2021. PMID: 33675873 No abstract available.
-
Reply to: "Myofibroblast YAP/TAZ is dispensable for liver fibrosis in mice".J Hepatol. 2021 Jul;75(1):241-243. doi: 10.1016/j.jhep.2021.04.014. Epub 2021 Apr 20. J Hepatol. 2021. PMID: 33892005 No abstract available.
References
-
- Duffy JP, Kao K, Ko CY et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010;252:652–661. - PubMed
-
- Evans HM, Kelly DA, McKiernan PJ et al. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology 2006;43:1109–1117. - PubMed

