A Novel Mu-Delta Opioid Agonist Demonstrates Enhanced Efficacy With Reduced Tolerance and Dependence in Mouse Neuropathic Pain Models
- PMID: 31201990
- PMCID: PMC6906261
- DOI: 10.1016/j.jpain.2019.05.017
A Novel Mu-Delta Opioid Agonist Demonstrates Enhanced Efficacy With Reduced Tolerance and Dependence in Mouse Neuropathic Pain Models
Abstract
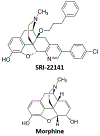
Numerous studies have demonstrated a physiological interaction between the mu opioid receptor (MOR) and delta opioid receptor (DOR) systems. A few studies have shown that dual MOR-DOR agonists could be beneficial, with reduced tolerance and addiction liability, but are nearly untested in chronic pain models, particularly neuropathic pain. In this study, we tested the MOR-DOR agonist SRI-22141 in mice in the clinically relevant models of HIV Neuropathy and Chemotherapy-Induced Peripheral Neuropathy (CIPN). SRI-22141 was more potent than morphine in the tail flick pain test and had equal or enhanced efficacy versus morphine in both neuropathic pain models, with significantly reduced tolerance. SRI-22141 also produced no jumping behavior during naloxone-precipitated withdrawal in CIPN or naïve mice, suggesting that SRI-22141 produces little to no dependence. SRI-22141 also reduced tumor necrosis factor-α and cyclooxygenase-2 in CIPN in the spinal cord, suggesting an anti-inflammatory mechanism of action. The DOR-selective antagonist naltrindole strongly reduced CIPN efficacy and anti-inflammatory activity in the spinal cord, without affecting tail flick antinociception, suggesting the importance of DOR activity in these models. Overall, these results provide compelling evidence that MOR-DOR agonists could have strong efficacy with reduced side effects and an anti-inflammatory mechanism in the treatment of neuropathic pain. PERSPECTIVE: This study demonstrates that a MOR-DOR dual agonist given chronically in chronic neuropathic pain models has enhanced efficacy with strongly reduced tolerance and dependence, with a further anti-inflammatory effect in the spinal cord. This suggests that MOR-DOR dual agonists could be effective treatments for neuropathic pain with reduced side effects.
Keywords: HIV neuropathy; Mu opioid receptor; chemotherapy-induced neuropathy; delta opioid receptor; dependence; neuroinflammation; tolerance.
Copyright © 2019 United States Association for the Study of Pain, Inc. Published by Elsevier Inc. All rights reserved.
Figures
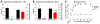
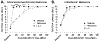
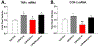
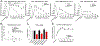
Similar articles
-
In vivo characterization of MMP-2200, a mixed δ/μ opioid agonist, in mice.J Pharmacol Exp Ther. 2011 Mar;336(3):767-78. doi: 10.1124/jpet.110.172866. Epub 2010 Nov 30. J Pharmacol Exp Ther. 2011. PMID: 21118955 Free PMC article.
-
In vivo pharmacological characterization of SoRI 9409, a nonpeptidic opioid mu-agonist/delta-antagonist that produces limited antinociceptive tolerance and attenuates morphine physical dependence.J Pharmacol Exp Ther. 2001 May;297(2):597-605. J Pharmacol Exp Ther. 2001. PMID: 11303048
-
Novel fentanyl-based dual μ/δ-opioid agonists for the treatment of acute and chronic pain.Life Sci. 2013 Dec 18;93(25-26):1010-6. doi: 10.1016/j.lfs.2013.09.016. Epub 2013 Sep 29. Life Sci. 2013. PMID: 24084045 Free PMC article.
-
Why mu-opioid agonists have less analgesic efficacy in neuropathic pain?Eur J Pain. 2019 Mar;23(3):435-454. doi: 10.1002/ejp.1328. Epub 2018 Nov 25. Eur J Pain. 2019. PMID: 30318675 Review.
-
Biased Opioid Ligands.Molecules. 2020 Sep 16;25(18):4257. doi: 10.3390/molecules25184257. Molecules. 2020. PMID: 32948048 Free PMC article. Review.
Cited by
-
Behavioral pharmacology of the mixed-action delta-selective opioid receptor agonist BBI-11008: studies on acute, inflammatory and neuropathic pain, respiration, and drug self-administration.Psychopharmacology (Berl). 2020 Apr;237(4):1195-1208. doi: 10.1007/s00213-019-05449-z. Epub 2020 Jan 7. Psychopharmacology (Berl). 2020. PMID: 31912192 Free PMC article.
-
Novel N-Substituted Benzomorphan-Based Compounds: From MOR-Agonist/DOR-Antagonist to Biased/Unbiased MOR Agonists.ACS Med Chem Lett. 2020 Jan 28;11(5):678-685. doi: 10.1021/acsmedchemlett.9b00549. eCollection 2020 May 14. ACS Med Chem Lett. 2020. PMID: 32435370 Free PMC article.
-
Novel N-normetazocine Derivatives with Opioid Agonist/Sigma-1 Receptor Antagonist Profile as Potential Analgesics in Inflammatory Pain.Molecules. 2022 Aug 12;27(16):5135. doi: 10.3390/molecules27165135. Molecules. 2022. PMID: 36014375 Free PMC article.
-
Elucidation on the In Vivo Activity of the Bivalent Opioid Peptide MACE2 against Several Types of Chronic Pain.ACS Omega. 2024 Nov 1;9(45):45214-45220. doi: 10.1021/acsomega.4c06449. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554412 Free PMC article.
-
Inhibiting spinal cord-specific hsp90 isoforms reveals a novel strategy to improve the therapeutic index of opioid treatment.Sci Rep. 2024 Jun 26;14(1):14715. doi: 10.1038/s41598-024-65637-6. Sci Rep. 2024. PMID: 38926482 Free PMC article.
References
-
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 258:299–303, 1991 - PubMed
-
- Ananthan S, Kezar HS 3rd, Carter RL, Saini SK, Rice KC, Wells JL, Davis P, Xu H, Dersch CM, Bilsky EJ, Porreca F, Rothman RB. Synthesis, opioid receptor binding, and biological activities of naltrexone-derived pyrido- and pyrimidomorphinans. J Med Chem. 42:3527–3538, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

