Telomerase structures and regulation: shedding light on the chromosome end
- PMID: 31202023
- PMCID: PMC8240480
- DOI: 10.1016/j.sbi.2019.04.009
Telomerase structures and regulation: shedding light on the chromosome end
Abstract
During genome replication, telomerase adds repeats to the ends of chromosomes to balance the loss of telomeric DNA. The regulation of telomerase activity is of medical relevance, as it has been implicated in human diseases such as cancer, as well as in aging. Until recently, structural information on this enzyme that would facilitate its clinical manipulation had been lacking due to telomerase very low abundance in cells. Recent cryo-EM structures of both the human and Tetrahymena thermophila telomerases have provided a picture of both the shared catalytic core of telomerase and its interaction with species-specific factors that play different roles in telomerase RNP assembly and function. We discuss also progress toward an understanding of telomerase RNP biogenesis and telomere recruitment from recent studies.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declarations of interest: none
Figures
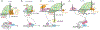
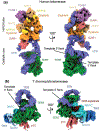
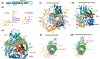

Similar articles
-
The architecture of Tetrahymena telomerase holoenzyme.Nature. 2013 Apr 11;496(7444):187-92. doi: 10.1038/nature12062. Epub 2013 Apr 3. Nature. 2013. PMID: 23552895 Free PMC article.
-
Structure of Telomerase with Telomeric DNA.Cell. 2018 May 17;173(5):1179-1190.e13. doi: 10.1016/j.cell.2018.04.038. Cell. 2018. PMID: 29775593 Free PMC article.
-
Progress in structural studies of telomerase.Curr Opin Struct Biol. 2014 Feb;24:115-24. doi: 10.1016/j.sbi.2014.01.008. Epub 2014 Feb 4. Curr Opin Struct Biol. 2014. PMID: 24508601 Free PMC article. Review.
-
Structural Biology of Telomerase.Cold Spring Harb Perspect Biol. 2019 Dec 2;11(12):a032383. doi: 10.1101/cshperspect.a032383. Cold Spring Harb Perspect Biol. 2019. PMID: 31451513 Free PMC article. Review.
-
Structures of telomerase at several steps of telomere repeat synthesis.Nature. 2021 May;593(7859):454-459. doi: 10.1038/s41586-021-03529-9. Epub 2021 May 12. Nature. 2021. PMID: 33981033 Free PMC article.
Cited by
-
The evolution of metazoan shelterin.Genes Dev. 2021 Dec 1;35(23-24):1625-1641. doi: 10.1101/gad.348835.121. Epub 2021 Nov 11. Genes Dev. 2021. PMID: 34764137 Free PMC article.
-
Structural biology of human telomerase: progress and prospects.Biochem Soc Trans. 2021 Nov 1;49(5):1927-1939. doi: 10.1042/BST20200042. Biochem Soc Trans. 2021. PMID: 34623385 Free PMC article. Review.
-
Human Telomerase RNA: Telomerase Component or More?Biomolecules. 2020 Jun 6;10(6):873. doi: 10.3390/biom10060873. Biomolecules. 2020. PMID: 32517215 Free PMC article. Review.
-
Evaluation of dyskerin expression and the Cajal body protein WRAP53β as potential prognostic markers for patients with primary vaginal carcinoma.Oncol Lett. 2022 Jan;23(1):30. doi: 10.3892/ol.2021.13148. Epub 2021 Nov 23. Oncol Lett. 2022. PMID: 34868367 Free PMC article.
-
Molecular and Evolutionary Analysis of RNA-Protein Interactions in Telomerase Regulation.Noncoding RNA. 2024 Jun 18;10(3):36. doi: 10.3390/ncrna10030036. Noncoding RNA. 2024. PMID: 38921833 Free PMC article. Review.
References
-
- Blackburn EH, Greider CW, Szostak JW: Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med 2006, 12:1133–1138. - PubMed
-
- Aubert G: Telomere dynamics and aging. Prog Mol Biol Transl Sci 2014, 125:89–111. - PubMed
-
- Collins K, Mitchell JR: Telomerase in the human organism. Oncogene 2002, 21:564. - PubMed

