Impaired neurodevelopmental pathways in autism spectrum disorder: a review of signaling mechanisms and crosstalk
- PMID: 31202261
- PMCID: PMC6571119
- DOI: 10.1186/s11689-019-9268-y
Impaired neurodevelopmental pathways in autism spectrum disorder: a review of signaling mechanisms and crosstalk
Abstract
Background: The development of an autistic brain is a highly complex process as evident from the involvement of various genetic and non-genetic factors in the etiology of the autism spectrum disorder (ASD). Despite being a multifactorial neurodevelopmental disorder, autistic patients display a few key characteristics, such as the impaired social interactions and elevated repetitive behaviors, suggesting the perturbation of specific neuronal circuits resulted from abnormal signaling pathways during brain development in ASD. A comprehensive review for autistic signaling mechanisms and interactions may provide a better understanding of ASD etiology and treatment.
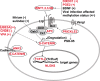
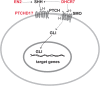
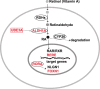
Main body: Recent studies on genetic models and ASD patients with several different mutated genes revealed the dysregulation of several key signaling pathways, such as WNT, BMP, SHH, and retinoic acid (RA) signaling. Although no direct evidence of dysfunctional FGF or TGF-β signaling in ASD has been reported so far, a few examples of indirect evidence can be found. This review article summarizes how various genetic and non-genetic factors which have been reported contributing to ASD interact with WNT, BMP/TGF-β, SHH, FGF, and RA signaling pathways. The autism-associated gene ubiquitin-protein ligase E3A (UBE3A) has been reported to influence WNT, BMP, and RA signaling pathways, suggesting crosstalk between various signaling pathways during autistic brain development. Finally, the article comments on what further studies could be performed to gain deeper insights into the understanding of perturbed signaling pathways in the etiology of ASD.
Conclusion: The understanding of mechanisms behind various signaling pathways in the etiology of ASD may help to facilitate the identification of potential therapeutic targets and design of new treatment methods.
Keywords: Autism spectrum disorder; BMP/TGF-β; FGF; Neurodevelopmental disorders; Retinoic acid (RA); SHH; Signaling crosstalk; WNT.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Potential crosstalk between sonic hedgehog-WNT signaling and neurovascular molecules: Implications for blood-brain barrier integrity in autism spectrum disorder.J Neurochem. 2021 Oct;159(1):15-28. doi: 10.1111/jnc.15460. Epub 2021 Aug 6. J Neurochem. 2021. PMID: 34169527 Free PMC article. Review.
-
Dysregulation of Multiple Signaling Neurodevelopmental Pathways during Embryogenesis: A Possible Cause of Autism Spectrum Disorder.Cells. 2021 Apr 20;10(4):958. doi: 10.3390/cells10040958. Cells. 2021. PMID: 33924211 Free PMC article. Review.
-
Overlapping Molecular Pathways Leading to Autism Spectrum Disorders, Fragile X Syndrome, and Targeted Treatments.Neurotherapeutics. 2021 Jan;18(1):265-283. doi: 10.1007/s13311-020-00968-6. Epub 2020 Nov 19. Neurotherapeutics. 2021. PMID: 33215285 Free PMC article. Review.
-
Delineating the Common Biological Pathways Perturbed by ASD's Genetic Etiology: Lessons from Network-Based Studies.Int J Mol Sci. 2017 Apr 14;18(4):828. doi: 10.3390/ijms18040828. Int J Mol Sci. 2017. PMID: 28420080 Free PMC article. Review.
-
Valproic acid exposure sequentially activates Wnt and mTOR pathways in rats.Mol Cell Neurosci. 2016 Sep;75:27-35. doi: 10.1016/j.mcn.2016.06.004. Epub 2016 Jun 23. Mol Cell Neurosci. 2016. PMID: 27343825
Cited by
-
Identification of Immune Infiltration and Iron Metabolism-Related Subgroups in Autism Spectrum Disorder.J Mol Neurosci. 2024 Jan 18;74(1):12. doi: 10.1007/s12031-023-02179-y. J Mol Neurosci. 2024. PMID: 38236354
-
A Mini-Review Regarding the Modalities to Study Neurodevelopmental Disorders-Like Impairments in Zebrafish-Focussing on Neurobehavioural and Psychological Responses.Brain Sci. 2022 Aug 28;12(9):1147. doi: 10.3390/brainsci12091147. Brain Sci. 2022. PMID: 36138883 Free PMC article. Review.
-
Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective.Front Psychiatry. 2022 Jul 14;13:911770. doi: 10.3389/fpsyt.2022.911770. eCollection 2022. Front Psychiatry. 2022. PMID: 35911241 Free PMC article. Review.
-
Potential crosstalk between sonic hedgehog-WNT signaling and neurovascular molecules: Implications for blood-brain barrier integrity in autism spectrum disorder.J Neurochem. 2021 Oct;159(1):15-28. doi: 10.1111/jnc.15460. Epub 2021 Aug 6. J Neurochem. 2021. PMID: 34169527 Free PMC article. Review.
-
Developmental exposure to pesticides that disrupt retinoic acid signaling causes persistent retinoid and behavioral dysfunction in zebrafish.Toxicol Sci. 2024 Mar 26;198(2):246-259. doi: 10.1093/toxsci/kfae001. Toxicol Sci. 2024. PMID: 38237923 Free PMC article.

