Immunometabolism: A new target for improving cancer immunotherapy
- PMID: 31202359
- PMCID: PMC6822174
- DOI: 10.1016/bs.acr.2019.03.004
Immunometabolism: A new target for improving cancer immunotherapy
Abstract
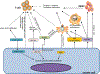
Fundamental metabolic pathways are essential for mammalian cells to provide energy, precursors for biosynthesis of macromolecules, and reducing power for redox regulation. While dysregulated metabolism (e.g., aerobic glycolysis also known as the Warburg effect) has long been recognized as a hallmark of cancer, recent discoveries of metabolic reprogramming in immune cells during their activation and differentiation have led to an emerging concept of "immunometabolism." Considering the recent success of cancer immunotherapy in the treatment of several cancer types, increasing research efforts are being made to elucidate alterations in metabolic profiles of cancer and immune cells during their interplays in the setting of cancer progression and immunotherapy. In this review, we summarize recent advances in studies of metabolic reprogramming in cancer as well as differentiation and functionality of various immune cells. In particular, we will elaborate how distinct metabolic pathways in the tumor microenvironment cause functional impairment of immune cells and contribute to immune evasion by cancer. Lastly, we highlight the potential of metabolically reprogramming the tumor microenvironment to promote effective and long-lasting antitumor immunity for improved immunotherapeutic outcomes.
Keywords: Antitumor immunity; Cancer immunotherapy; Immune checkpoints; Immunometabolism; Tumor microenvironment.
© 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Competing interests
None declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

