The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction
- PMID: 31202947
- PMCID: PMC6669893
- DOI: 10.1016/j.jcin.2019.03.035
The BASILICA Trial: Prospective Multicenter Investigation of Intentional Leaflet Laceration to Prevent TAVR Coronary Obstruction
Abstract
Objectives: The BASILICA (Bioprosthetic or native Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction during TAVR) investigational device exemption trial was a prospective, multicenter, single-arm safety and feasibility study.
Background: Coronary artery obstruction is a rare but devastating complication of transcatheter aortic valve replacement (TAVR). Current stent-based preventative strategies are suboptimal. Bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction during TAVR (BASILICA) is a novel transcatheter technique performed immediately before TAVR to prevent coronary artery obstruction.
Methods: Subjects with severe native or bioprosthetic aortic valve disease at high or extreme risk for surgery, and at high risk of coronary artery obstruction, were included. The primary success endpoint was successful BASILICA and TAVR without coronary obstruction or reintervention. The primary safety endpoint was freedom from major adverse cardiovascular events. Data were independently monitored. Endpoints were independently adjudicated. A core laboratory analyzed computed tomography images.
Results: Between February 2018 and July 2018, 30 subjects were enrolled. Primary success was met in 28 (93%) subjects. BASILICA traversal and laceration was successful in 35 of 37 (95%) attempted leaflets. There was 100% freedom from coronary obstruction and reintervention. Primary safety was met in 21 (70%), driven by 6 (20%) major vascular complications related to TAVR but not BASILICA. There was 1 death at 30 days. There was 1 (3%) disabling stroke and 2 (7%) nondisabling strokes. Transient hemodynamic compromise was rare (7%) and resolved promptly with TAVR.
Conclusions: BASILICA was feasible in both native and bioprosthetic valves. Hemodynamic compromise was uncommon. Safety was acceptable and needs confirmation in larger studies. BASILICA appears effective in preventing coronary artery obstruction from TAVR in subjects at high risk.
Keywords: bioprosthetic heart valve failure; coronary artery obstruction; structural heart disease; transcatheter aortic valve replacement; transcatheter electrosurgery.
Published by Elsevier Inc.
Figures


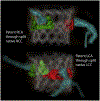
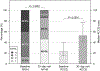

Comment in
-
The Splitting of Leaflets to Prevent Coronary Occlusion During TAVR.JACC Cardiovasc Interv. 2019 Jul 8;12(13):1253-1255. doi: 10.1016/j.jcin.2019.04.009. Epub 2019 Jun 12. JACC Cardiovasc Interv. 2019. PMID: 31202952 No abstract available.
References
-
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016; 374:1609–20. - PubMed
-
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017; 376:1321–31. - PubMed
-
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018;72:2095–105. - PubMed
-
- Ribeiro HB, Webb JG, Makkar RR, et al. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. J Am Coll Cardiol 2013;62:1552–62. - PubMed
-
- Ribeiro HB, Rodes-Cabau J, Blanke P, et al. Incidence, predictors, and clinical outcomes of coronary obstruction following transcatheter aortic valve replacement for degenerative bioprosthetic surgical valves: insights from the VIVID registry. Eur Heart J 2018;39:687–95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

