Molidustat for the treatment of renal anaemia in patients with non-dialysis-dependent chronic kidney disease: design and rationale of two phase III studies
- PMID: 31203242
- PMCID: PMC6588957
- DOI: 10.1136/bmjopen-2018-026704
Molidustat for the treatment of renal anaemia in patients with non-dialysis-dependent chronic kidney disease: design and rationale of two phase III studies
Abstract
Introduction: Anaemia is a common complication of chronic kidney disease (CKD). Owing to the limitations of erythropoiesis-stimulating agents (ESAs), the current standard of care, there is a need to develop new therapies. Hypoxia-inducible factor prolyl-hydroxylase (HIF-PH) inhibitors might be a promising new treatment option. Molidustat is an oral HIF-PH inhibitor that stimulates the endogenous, predominantly renal, production of erythropoietin and was generally well tolerated in phase IIb clinical trials. Here, we report the design and rationale of two studies from the molidustat phase III programme: MolIdustat once dailY improves renal Anaemia By Inducing erythropoietin (MIYABI).
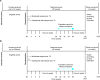
Methods and analysis: MIYABI Non-Dialysis-Correction (ND-C) and MIYABI Non-Dialysis-Maintenance (ND-M) are randomised, open-label, parallel-group, multicentre studies that aim to demonstrate the efficacy of molidustat treatment compared with darbepoetin alfa in patients with anaemia and non-dialysis-dependent CKD. The secondary objectives are to assess the safety, pharmacokinetics and pharmacodynamics of molidustat treatment. MIYABI ND-C will recruit patients currently untreated with ESAs, whereas patients treated with an ESA will enter MIYABI ND-M. Each study will recruit 150 patients who will be randomised in a 1:1 ratio to receive either molidustat or darbepoetin alfa for 52 weeks, with efficacy evaluated during weeks 30-36. Study drug doses will be titrated regularly using an interactive voice/web response system with the aim of maintaining the patients' haemoglobin (Hb) levels between ≥110 and <130 g/L. The primary objective will be achieved if, in molidustat-treated patients, the mean Hb level remains within the target range during the evaluation period, and if the change in the mean Hb level at evaluation time points from baseline is non-inferior to darbepoetin alfa.
Ethics and dissemination: The protocols were approved by ethics committees at all participating sites. These studies will be conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. Results arising from these studies will be published in peer-reviewed journal(s).
Trial registration numbers: NCT03350321; Pre-results, NCT03350347; Pre-results.
Keywords: chronic kidney disease; molidustat; renal anaemia.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MT, YM, KI and TY are employees of Bayer Yakuhin Ltd. TA received consulting and lecture fees from Bayer Yakuhin Ltd during the conduct of the study. TA has also received consulting, lecture or manuscript fees outside the submitted work from Astellas, GlaxoSmithKline, JT Pharmaceuticals, Kissei Pharmaceutical Co. Ltd, Kyowa Hakko Kirin, Nipro Corporation, Fuso Pharmaceutical Industries Ltd, Ono Pharmaceutical Co. Ltd, Chugai Pharmaceutical Co. Ltd and Torii Pharmaceutical Co. Ltd. HY received consulting fees from Bayer Yakuhin Ltd during the conduct of the study.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
